
Page 31
conferenceseries
.com
Volume 4
Toxicology: Open Access
ISSN: 2476-2067
Toxicology Congress 2018
March 12-14, 2018
March 12-14, 2018 Singapore
14
th
World Congress on
Toxicology and Pharmacology
Tissue distribution of Suvorexant in three postmortem cases
Brian Waters, Kenji Hara, Natsuki Ikematsu, Mio Takayama, Aya Matsusue, Masayuki Kashiwagi and Shin-ichi Kubo
Fukuoka University, Japan
Statement of the Problem:
Suvorexant (Belsomra®) is a relatively new
insomnia medication that has been available in the US and Japan since 2014.
It is a dual orexin receptor antagonist that promotes sleep by inhibiting the
binding of orexin neurons to the OX1R and OX2R receptors. In this report, we
describe the detection and quantitation of Suvorexant from the postmortem
specimens of three recent autopsy cases handled by our department.
Methodology & Theoretical Orientation:
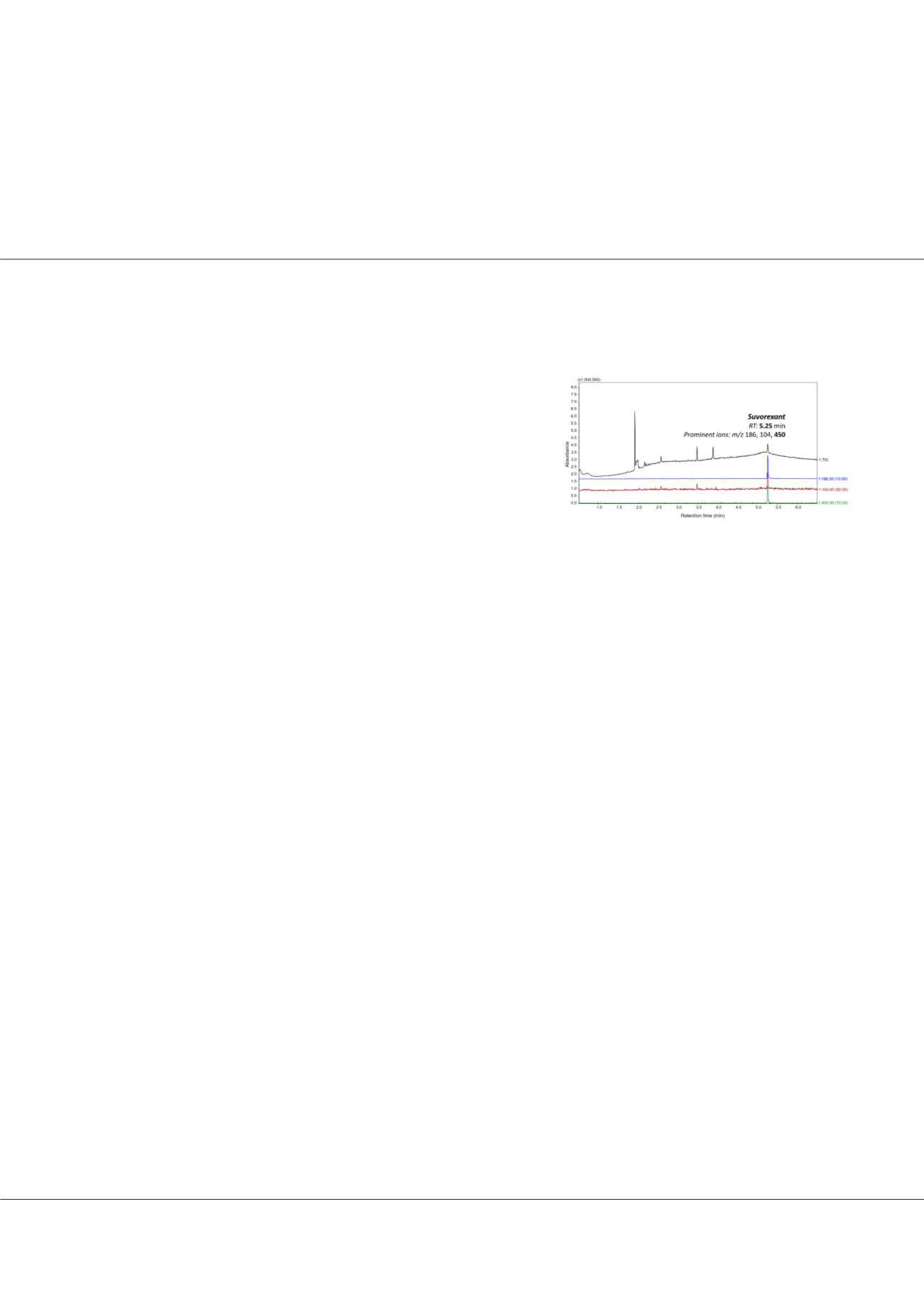
Suvorexant was identified by fast
GC-MS during routine screening and quantitated by a fully validated LC-MS/
MS method. Quantitation was achieved by positive electrospray ionization
in the selected reaction monitoring mode. Monitored transitions were m/z
451>186 for quantitation and m/z 451>104 for qualification. Diazepam-d5 was used as an internal standard.
Findings:
Suvorexant was detected and quantitated in the body fluids and tissues of three autopsy cases. The specimens
included cardiac blood, peripheral blood, urine, liver, kidney, spleen, pancreas, lung, muscle, fat and cerebrospinal fluid. Tissue
distribution across the three cases will be presented and discussed.
Conclusion & Significance:
The use of Suvorexant as an insomnia medication has recently increased around the world. To
our knowledge this is the first instance of Suvorexant being quantitated from actual autopsy cases. It is possible the presence
of this medication in clinical and forensic samples has been missed due to its high boiling point and thus late elution in gas
chromatography. We were able to detect Suvorexant in three cases by using fast GC-MS which significantly reduced its retention
time. It is likely that this compound will be encountered more often by the forensic and clinical toxicology communities going
forward.
Recent Publications
1. Hara K, Waters B, Ikematsu N, et al. (2016) Development of a preparation method to produce a single sample that can be
applied to both LC—MS/MS and GC—MS for the screening of postmortem specimens.
Legal Medicine
; 21: 85-92.
2. Waters B, Ikematsu N, Hara K, et al. (2016) GC-PCI-MS/MS and LC-ESI-MS/MS databases for the detection of 104
psychotropic compounds.
Legal Medicine
; 20: 1-7.
References
1. Carson M, Kerrigan S (2017) Quantification of Suvorexant in urine using gas chromatography/mass spectrometry.
Journal
of Chromatography B
; 1040: 289-294.
2. Iqbal M, Ezzeldin E, Khalil N, Al-Rashood S, Al-Rashood K (2017) Simple and Highly Sensitive UPLC-ESI-MS/MS Assay
for Rapid Determination of Suvorexant in Plasma.
Journal of Analytical Toxicology
; 41: 114-120.
Biography
Brian Waters has received his Master of Science degree in Criminalistics from California State University Los Angeles, USA. After working as a Criminalist for
the County of Los Angeles, Department of Coroner/Medical Examiner for almost eight years, he accepted a position as an Assistant Professor in the Department
of Forensic Medicine at Fukuoka University in Japan. His specialty is postmortem forensic toxicology and he has published academic papers on fast gas
chromatography-mass spectrometry, the analysis of novel psychoactive compounds, preparation methods for postmortem samples and the analysis of volatile
hydrocarbons in blood.
bwaters@fukuoka-u.ac.jpBrian Waters et al., Toxicol Open Access 2018, Volume 4
DOI: 10.4172/2476-2067-C1-005
Figure-1:
A GC-MS chromatogram of Suvorexant
extracted from an autopsy blood sample.
















