
Notes:
Page 68
Chromatography 2016
September 21-23, 2016
Volume 7, Issue 5(Suppl)
J Chromatogr Sep Tech 2016
ISSN: 2157-7064 JCGST, an open access journal
conferenceseries
.com
September 21-23, 2016 Amsterdam, Netherlands
World Congress on
Chromatography
Simultaneous determination and validation of telmisartan and amlodipine in pharmaceutical preparations
using capillary electrophoretic method
Ebru TÜRKÖZ Acar
1
, Hayati ÇELiK
1
, Sevinç KURBANOGLU
2
, Mehmet GÜMÜSTAS
2
and Sibel A OZKAN
2
1
Yeditepe University, Turkey
2
Ankara University, Turkey
C
ardiovascular diseases (CVDs) are the disorders of heart and blood vessels and primarily include coronary heart disease,
hypertension, cerebrovascular disease, peripheral artery disease, rheumatic heart disease, congenital heart disease and
heart failure. CVDs are the major cause of death in developed countries and also are rapidly emerging as a main cause of death
in the developing World. The major risk factors involved in CVDs are high low density lipoprotein (LDL) cholesterol, raised
blood pressure, increased serum homocysteine level and platelet aggregation, which are primarily caused by unhealthy diet,
physical inactivity and tobacco use. There are various pharmaceutical formulations containing different active materials. One
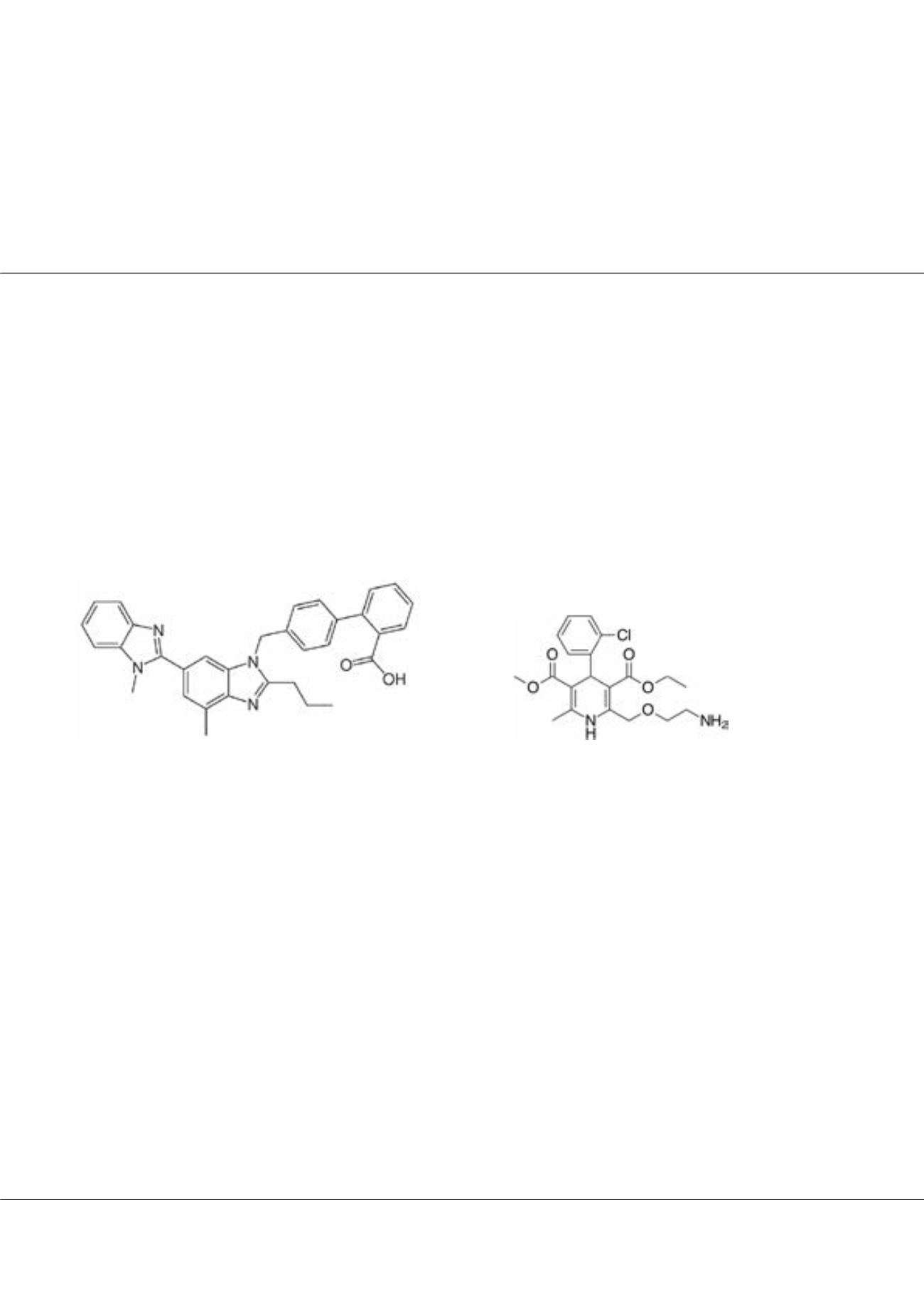
of them contains Telmisartan and Amlodipine besylate. Telmisartan is an angiotensin II receptor (type AT1) antagonist used
in the management of hypertension. It is prevents the constriction (narrowing) of blood vessels.
Telmisartan
Amlodipin
Amlodipine besylate is in a class of drugs called beta-blockers. Beta-blockers affect the heart and circulatory system (arteries
and veins). It is used to lower blood pressure, lower heart rate, reduce chest pain, and to reduce the risk of recurrent heart
attacks. In the literature there are different studies analyzing Telmisartan, and Amlodipine besylate hence, there is no capillary
electrophoresis method analyzing these drugs simultaneously. In this study a capillary electrophoretic method will be presented.
The aim of study determinates the telmisartan and amlodipin besylate, simultaneously, in tablet formulation. The proposed
method has been extensively validated in terms of precision, accuracy. Linear range, limit of detection and quantification
values, are also calculated and discussed according to ICH Guidelines and USP criteria. The method can be used for the
determination of Telmisartan and Amlodipine in their pharmaceutical preparations.
Biography
Ebru TÜRKÖZ Acar has completed his PhD from Ondokuz Mayıs University Science Institute Analytical Chemistry Department. She is a lecturer/researcher at
Yeditepe University, Faculty of Pharmacy, Analytical Chemistry department.
ebruturkozacar@gmail.comEbru TÜRKÖZ Acar et al., J Chromatogr Sep Tech 2016, 7:5(Suppl)
http://dx.doi.org/10.4172/2157-7064.C1.017















