
Page 156
Notes:
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Mario Daniel Garcia et al., J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0101
Acetohydroxyacid synthase regulation, structure and inhibition by commercial herbicides
Mario Daniel Garcia, Thierry Lonhienne
and
Luke Guddat
University of Queensland, Australia
A
cetohydroxyacid synthase (E.C. 2.2.1.6) is the first enzyme in the branched chain of amino acid biosynthesis pathway. It
is the target of five classes of commercial herbicides (i.e. sulfonylureas, imidazolinones, triazolopyrimidines, pyrimidinyl-
benzoates, sulfonylamino-carbonyl-triazolinones), which are popular amongst famers worldwide due to their extremely high
potency, low toxicity to animals and high selectivity for weeds over crops. Although AHAS is of high importance, some aspects
of the enzyme structure, function and inhibition have remained unresolved. Here we show that FAD reduction is required for
AHAS activity and that soluble quinone derivatives (e.g. ubiquinones) regulate this activity by oxidizing FAD and by a slow
process of FAD re-reduction. A new high-resolution structure of
Saccharomyces cerevisiae
AHAS (2 Å) reveals FAD is trapped
in two different conformations indicative of two oxidation states occurring at the same time. Moreover, this structure shows
the position of two oxygen molecules in the active site and an oxygen access channel. In addition, we have determined the
crystal structures of un-inhibited
Arabidopsis thaliana
AHAS and in complex with herbicides of the pyrimidinyl-benzoate and
sulfonylamino-carbonyl-triazolinone families. These structures show that the herbicide binding site in plant AHAS adopts a
folded state even in the absence of herbicide. This is unexpected because the equivalent regions in yeast AHAS are disordered
or have a different folding. These structures and mass spectrometry show that the herbicides trigger an alteration of the enzyme
cofactor thiamine diphosphate. Kinetic studies show that all five families of herbicides elicit accumulative inhibition of the
enzyme, which is linked to thiamin diphosphate degradation. These features contribute to the extraordinary potency of these
herbicides when in action.
Biography
Mario Daniel Garcia is in his third year of PhD studies at The University of Queensland, Australia. He obtained his Bachelor’s degree (Hons.) in Biotechnology at
Universidad de las Fuerzas Armadas, Ecuador, in 2010. His research work has focused on understanding the structure, function and inhibition of plant and yeast
acetohydroxy acid synthase, with a special interest in describing the role of commercial herbicides that target AHAS have in the degradation/modification of thiamin
diphosphate.
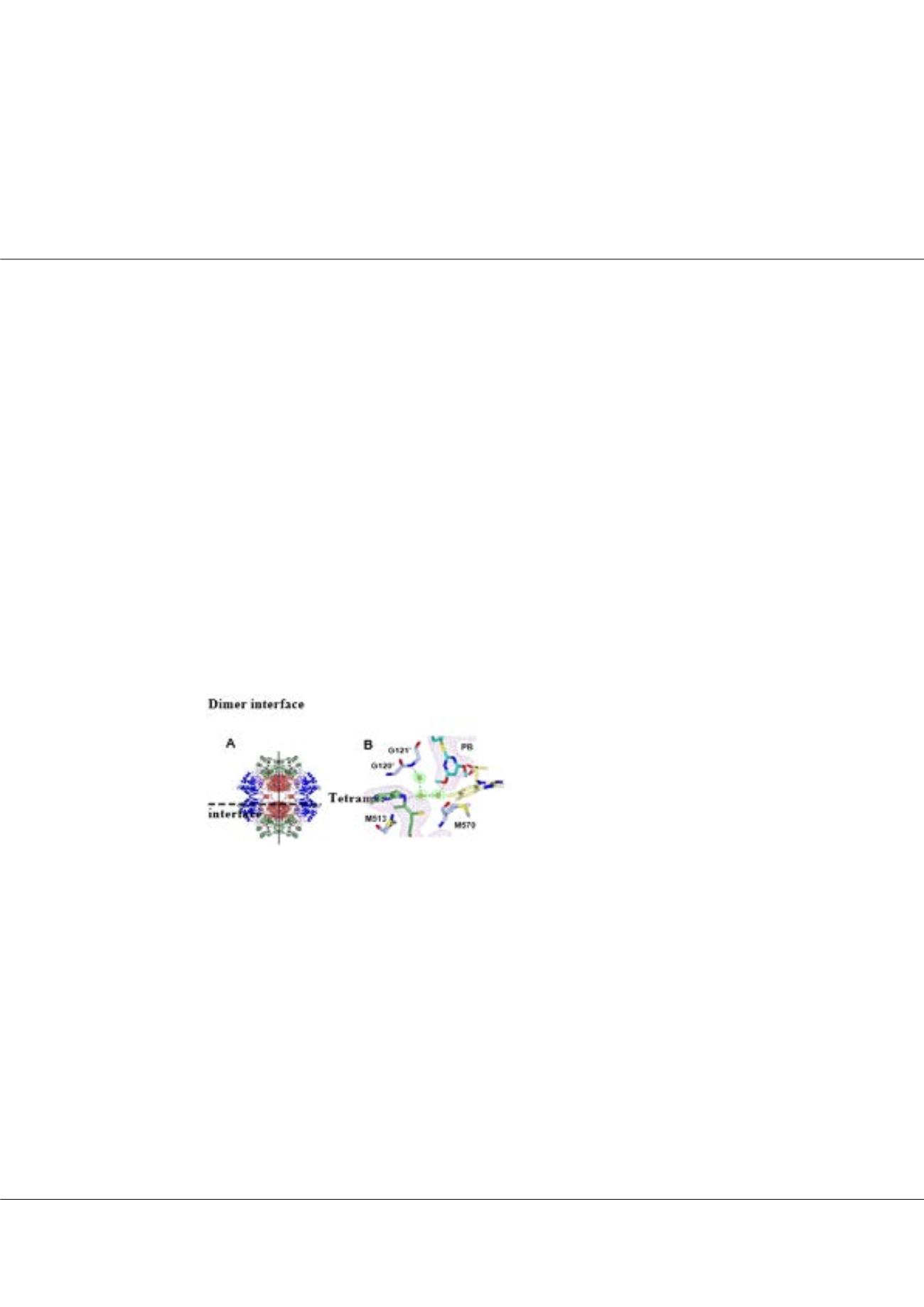
mario.garciasolis@uq.net.auFigure1:
(A) Crystal structure of A. thaliana AHAS3. (B) Herbicide
binding site of A. thaliana AHAS in complex with pyrithiobac,
showing the degradation of the thiamin diphosphate cofactor4.
















