
Page 166
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Strategic recovery of recombinant human truncated Cyclin A and Cyclin D in
Escherichia coli
for
characterization with putative inhibitors
Nikolopoulos Vaios, Grigoroudis Asterios
and
Kontopidis George
University of Thessaly, Greece
T
argeting cyclins enables us to interfere with cell cycle in order to inhibit the cancerous process which is the result of a non-
prober regulation of this cell cycle control1. Through cell cycle, Cyclin dependent kinases (CDKs) as well as their activation
partners, Cyclins, are regulators of progression and proliferation through activation of cell cycle checkpoints and inhibited
by CKIs, thus they have been widely held as anti-cancer targets2. This work attempts the over-expression of recombinant
truncated forms of human Cyclin A (His-tagged CCNA2) and Cyclin D (GST-tagged CCND1) seeking the high levels of
yield and purity for crystallization and ligand characterization purposes. Seeking to optimize binding affinity of CCNA2/
inhibitor and CCND1/inhibitor complexes, in order to develop more active inhibitors against CDks/Cyclins activity for cancer
research. CCNA2 and CCND1 fused to the appropriate bacterial vectors3, in E. coli, as one of the most widely used expression
hosts. Following heterologous over-expression, the recombinant proteins often fail to properly fold, resulting in formation of
insoluble aggregates (inclusion bodies - IB). It is known that co-expression with chaperone proteins facilitates their folding
process, while increasing solubility in a bacterial over-expression system4. Optimizing the high levels of yield and purity
required a number of strategies took place for the recombinant proteins. BL21 (DE3) expression host is preferred for both
recombinant proteins5. Homogenization increases the levels of regained protein from IB. Following denaturation of IB, Urea
buffer is suggested for refolding. CCNA2 and CCND1 refolding was more efficient with GSH/GSSG rather than DTT, while
stability of cyclins was achieved with elevated concentrations of MgCl2. In case of CCND1 co-expression with chaperone
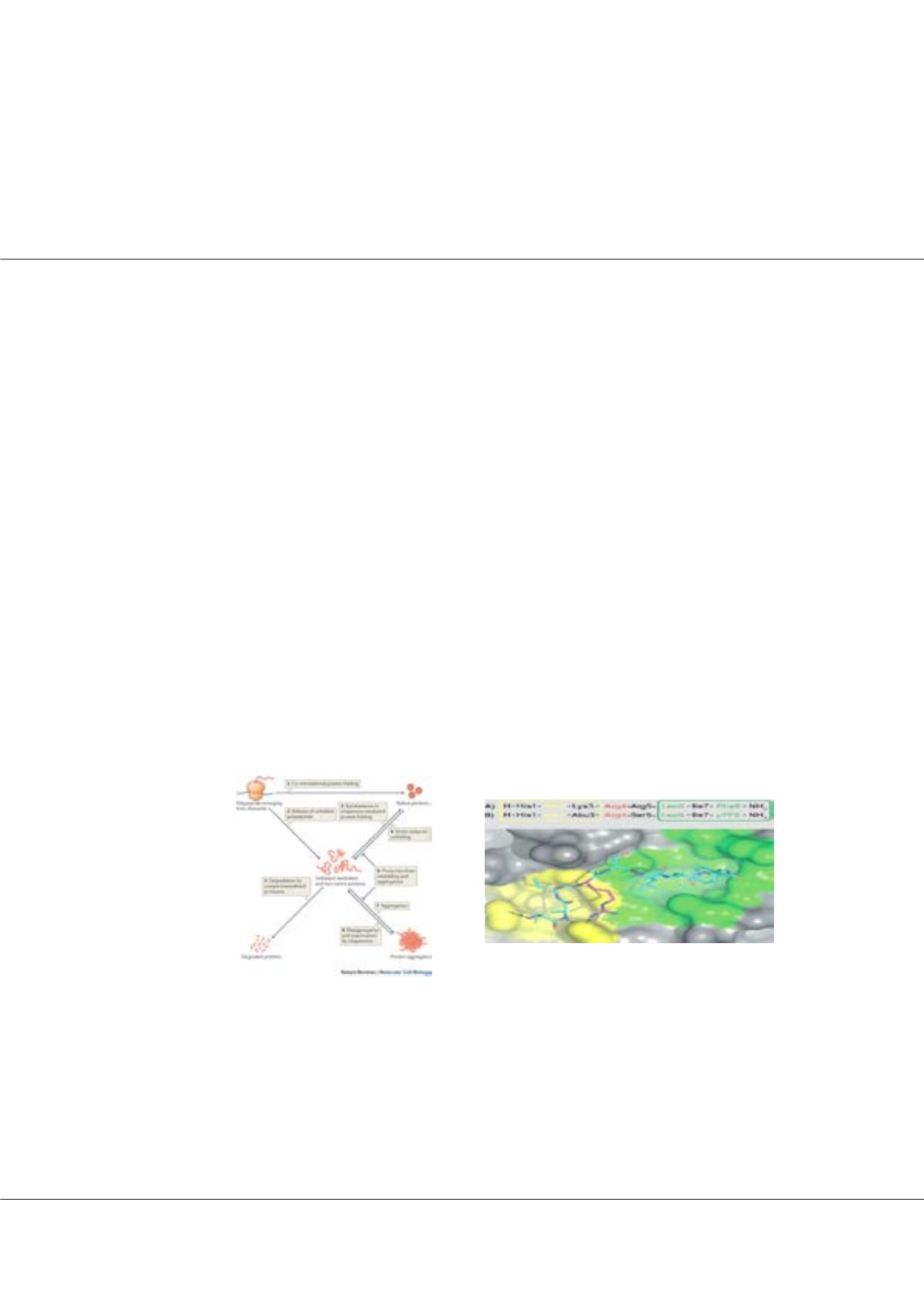
plasmid pTf16 increases substantially soluble protein. Existing synthesized peptides, designed with REPLACE (REplacement
with Partial Ligand Alternatives through Computational Enrichment). This structure-activity co-relation with non-fluorescent
peptides as cyclin groove putative inhibitors (CGI) where tested. Image
Biography
Vaios Nikolopoulos is a Biologist MSc and as a PhD candidate, has his expertise in molecular biology and diagnostics. His passion is to combine more aspects
and functions of molecules and their pathways in order to see the big picture in a pathologic state either this is a cancerous process or diabetes etc. Currently he
is working in protein purification and ligand binding studies while studying crystallography and also elaborates with other colleagues in various clinical studies. His
goal is to be a part of this “thinking tank” community to share his inquiring mind and to absorb new ideas in this highly competitive area of biosciences.
nikvaios@bio.uth.grNikolopoulos Vaios et al., J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0101
Figure2:
REPLACE strategy. N- terminal HAKRRLIF
binding motif from native CGI p21waf1, synthetic peptides
were designing while tested as putative inhibitors2
Figure1:
The protein quality control network6














