
Page 159
Notes:
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Jae-hee Jeong, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0101
Structural insghts into the histidine trimethylation activity of EgtD from
Mycobacterium smegmatis
Jae-hee Jeong
Pohang Accelerator Laboratory, Korea
E
gtD is an S-adenosyl-l-methionine (SAM)-dependent histidine N-methyltransferase that catalyzes the formation of
hercynine from histidine in the ergothioneine biosynthetic process of
Mycobacterium smegmatis
. Ergothioneine is a
secreted antioxidant that protects mycobacterium from oxidative stress. Here, we present three crystal structures of EgtD in
the apo form, the histidine-bound form, and the
S
-adenosyl-l-homocysteine (SAH)/histidine-bound form. The study revealed
that EgtD consists of two distinct domains: a typical methyltransferase domain and a unique substrate binding domain. The
histidine binding pocket of the substrate binding domain primarily recognizes the imidazole ring and carboxylate group of
histidine rather than the amino group, explaining the high selectivity for histidine and/or (mono-, di-) methylated histidine
as substrates. In addition, SAM binding to the MTase domain induced a conformational change in EgtD to facilitate the
methyl transfer reaction. The structural analysis provides insights into the putative catalytic mechanism of EgtD in a processive
trimethylation reaction.
Biography
Jae-hee Jeong is a Researcher in Structural Biology lab of Pohang Accelerator Laboratory. She has accumulated expertise in protein-protein interaction and
macromolecule X-ray crystallography after years of experience in research.
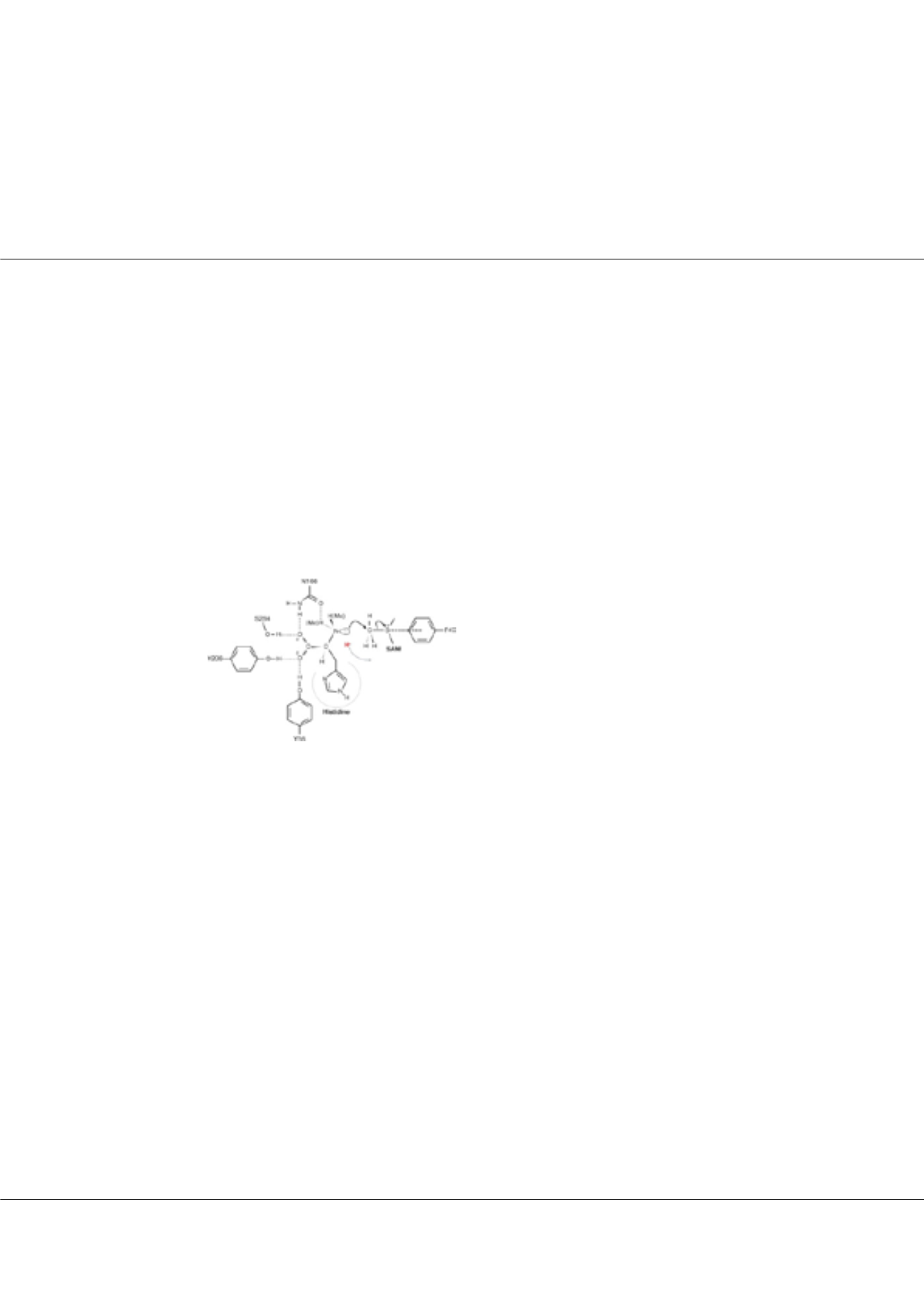
specials@postech.ac.krFigure1:
Scheme of the proposed speculative catalytic
mechanism of EgtD: The a-amino nitrogen of histidine is
aligned for a direct in-line SN2 nucleophilic attack by forming
hydrogen bonding interactions with the depicted residues. The
positively charged sulfonium ion of SAM will be stabilized by
the charge–p interaction with Phe47. The lone pair of electrons
from the nitrogen will be obtained after a proton loss to solvent,
which is indicated as a red letter and a dashed arrow during
the processive methylation reactions.
















