
Page 153
Notes:
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Su Youn Lee et al., J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0101
Conformational analysis of splicing-dependent regulation in tissue-specific NCX variants
Su Youn Lee
1
, Moshe Giladi
2
, Ka Young Chung
1
and
Daniel Khananshvili
2
1
Sungkyunkwan University, South Korea
2
Tel-Aviv University, Israel
T
issue-specific splice variants of Na
+
/Ca
2+
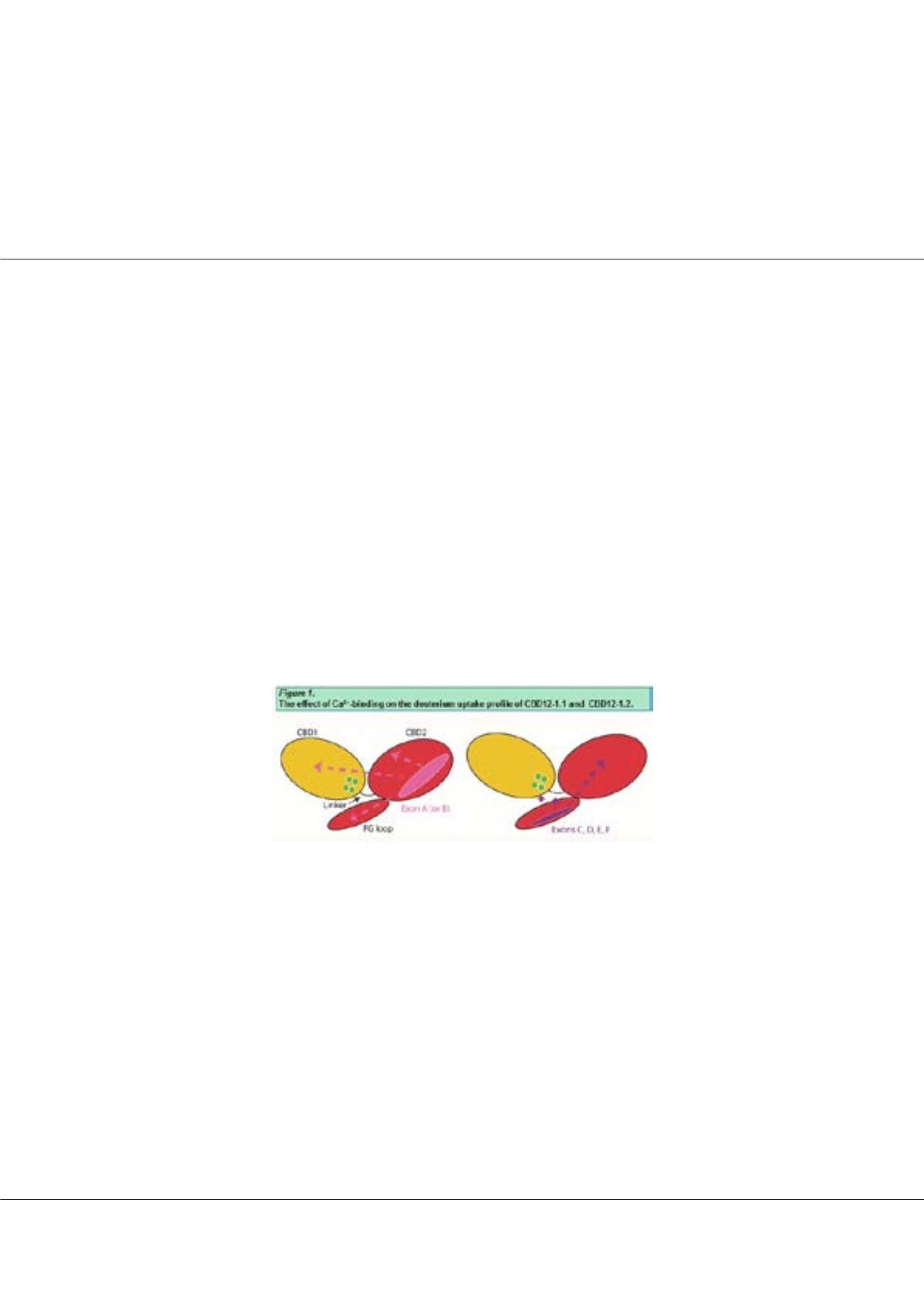
exchanger proteins (NCX1-3) contain two calcium-binding regulatory domains,
CBD1 and CBD2. CBD1 contains highly conserved allosteric Ca
2+
sensors, and CBD2 somehow controls their dynamic
properties. NCXs are activated with Ca
2+
interaction and Na
+
dependent inactivation is alleviated with Ca
2+
binding, where the
regulatory specificity is controlled by the splicing segment solely located on CBD2. Distinct regulatory specificities of splice
variants are promoted by certain combinations of two mutually exclusive exons (A, B) and of four cassette exons (C, D, E, F)
of CBD2, although the structure-dynamic nature remains unclear. Using hydrogen deuterium exchange – mass spectrometry
(HDX-MS), we investigated the effect of exons on CBDs backbone dynamics and found that the mutually exclusive exons A
and B stabilize interdomain interactions in apo-protein, where the exons differ in their capacity to predefine dynamic responses
to Ca
2+
binding. It was also observed that cassette exons gradually elongate CBD2 FG-loop, solidifying the interdomain Ca
2+
salt-bridge of the two-domain interface and secondarily modulating the Ca
2+
-bound states. The effects on Ca
2+
induced
conformational changes in matching splice variants correlate with Ca
2+
off-rates, while disclosing the local and distant effects
of structurally disordered/dynamic segments on the folded structures. Present findings are discussed considering the new
concepts explaining how the structurally disordered splicing segments can diversify regulatory specificities in tissue-specific
variants. Thus, the newly found dynamic feature of CBDs may represent a mechanical basis for diversifying the regulatory
feature in NCXs and similar proteins.
Biography
Su Youn Lee is currently studying the structures of drug-target proteins in her PhD program. She has been trained to study the structures of proteins using HDX-
MS, which provides information about the conformational change of proteins. She has collaborated with an expert in the NCX field and played a significant role
in a project which elaborated the dynamics and the structural mechanism of NCX regulation. And the results of this study have been published on major journals
(
Biochem J
2015
, FASEB J
2016, and
Scientific Reports
2017). Her study will contribute in suggesting a new NCX drug target sites, which will increase the
selectivity and effectiveness and reduce side effects of NCX targeting drugs.
youn3887@hanmail.net















