
Page 148
Notes:
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Searching drugs for NF-κB pathway regulation: BIR1 domains of IAPs as promising new targets
Luca Sorrentino
National Research Council, Italy
I
nhibitor of apoptosis proteins are target of extensive research in the field of cancer therapy since they control apoptosis and
cell survival. The most studied IAPs, namely XIAP, cIAP1 and cIAP2, were investigated for their ability to bind and inhibit
caspases, thus blocking apoptosis. However, IAPs interaction network is much more complicated as they interact with multiple
cellular partners to regulate the NF-kB signaling pathway, which is pivotal for cell survival. So far, the development of highly
active Smac-mimetics (SM) targeting type II BIR domains of IAPs was pursued by many research groups. Nevertheless, in
some cases, SM-mediated cIAP1 degradation leads to non-canonical NF-κB activation, by inducing cIAP
2
gene expression,
which overcomes cIAP1 absence and suppresses TNFα-mediated cell death. We propose to explore alternative mechanisms
that can be exploited to interfere with IAP-involving signaling cascades in NF-κB regulation. We focused our attention on IAPs'
type I BIR domains (BIR1), that lack the N-terminal peptide-binding groove necessary for SM-binding in type II BIR domains.
The BIR1 domains of cIAP2 and of XIAP interact with a TRAF1:(TRAF2)
2
heterotrimer and with TAB1, an upstream adaptor
for TAK1 kinase activation, respectively, to regulate the canonical NF-κB signaling. We investigated the protein-protein
interaction surfaces of IAPs' BIR1 domains finding that, in the crystal structures of cIAP
2
-BIR1/TRAFs and XIAP-BIR1/TAB1,
they display an identical interaction surface. This observation points out remarkable common features in IAPs-BIR1 domains
that can be exploited to identify a new class of molecules able to specifically inhibit type I BIR domains. In this context, we
identified the compound NF023 and solved the crystal structures of human XIAP-BIR1 domain in the absence and presence of
the inhibitor or of its analog cmp247. Furthermore, we identified several new molecules that could possibly inhibit XIAP-BIR1
homodimerization and that are currently under study.
Biography
Luca Sorrentino has always been interested in the study of proteins involved in the regulation of cell death or cell survival, two pivotal processes at the base of
cancer development. During his education and PhD program, he gained expertise in cloning, production and purification of recombinant proteins followed by
their thorough biochemical and structural characterization. Moreover, he also performed several experiments at the ESRF synchrotron in Grenoble. During the
last years, he participated in international conferences and was supervisor of several graduate students. He is currently post-doc in the laboratory of Dr. Eloise
Mastrangelo and Dr. Mario Milano (IBF-CNR, Milan, Italy), working on a project aimed at the structure-based design of a new class of compounds active in the
regulation of the NF-κB pathway.
luca.sorrentino88@yahoo.itLuca Sorrentino, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0101
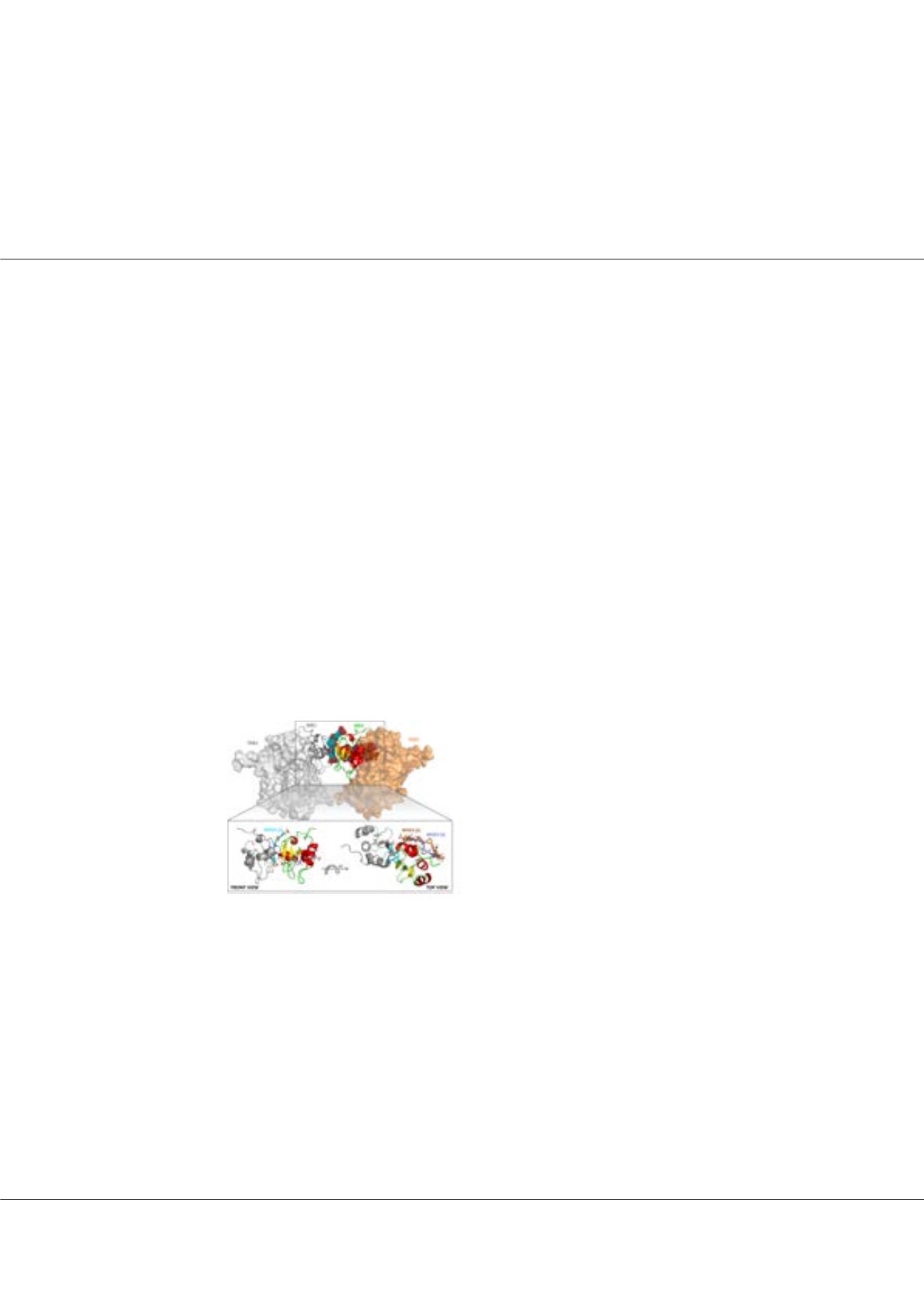
Figure1:
NF023 potentially impairs XIAP-BIR1 homodimerization.
Crystal structure of XIAP-BIR1 (colored cartoons) in the presence
of NF023 (in sticks), as superimposed with the crystal structure of
BIR1 in complex with TAB1 (colored surfaces, PDB code: 2POP).
The compound impairs XIAP-BIR1 homodimerization, possibly
destabilizing its interaction with TAB1.
















