
Page 64
conferenceseries
.com
Volume 8
Journal of Biotechnology & Biomaterials
ISSN: 2155-952X
Biotech Congress 2018 & Enzymology 2018
March 05-07, 2018
JOINT EVENT
20
th
Global Congress on
Biotechnology
3
rd
International Conference on
Enzymology and Molecular Biology
&
March 05-07, 2018 London, UK
The dual role of integrase in HIV-1 replication
Mamuka Kvaratskhelia
University of Colorado School of Medicine, USA
A
key HIV-1 enzyme integrase catalyzes irreversible insertion of a viral DNA copy of its RNA genome into human chromosome,
which is essential for viral replication. Therefore, integrase is an important therapeutic target. Productive integration into host
chromatin results in the formation of the strand transfer complex (STC) containing catalytically joined viral and target DNAs. We
have used cryo-EM coupled with biochemistry and virology experiments to obtain high-resolution structures for STCs and to
characterize the integrase multi-subunit assemblies into large, nucleoprotein complexes. We are currently extending these studies
to elucidate structural basis for the mode of action of clinically used integrase strand transfer inhibitors (INSTIs), which bind to the
enzyme active site in the context of the integrase-viral DNA complex and block the strand transfer reaction. Our parallel efforts are
focused on studying allosteric HIV-1 integrase inhibitors (ALLINIs), which are currently undergoing clinical trials (2-5). Unlike
INSTIs, ALLINIs bind at the integrase dimer interface and induce aberrant protein multimerization. Unexpectedly, in infected cells
ALLINIs were significantly more potent during virion maturation rather than during integration. ALLINIs markedly altered virus
particle morphogenesis by misplacing the ribonucleoprotein complexes outside the protective viral capsid shell and yielded inactive
virions. In turn, these findings have suggested that integrase has a second function in HIV-1 biology. Our follow up studies have
revealed that integrase directly binds the viral RNA genome in virions. These interactions have specificity, as integrase exhibits
distinct preference for select viral RNA structural elements. ALLINIs impair integrase binding to viral RNA in virions of wild-type,
but not escape mutant, virus. These results reveal an unexpected biological role of integrase binding to the viral RNA genome during
virion morphogenesis and elucidate the mode of action of ALLINIs. Collectively our findings indicate that viral integrase plays a dual
role during HIV-1 replication.
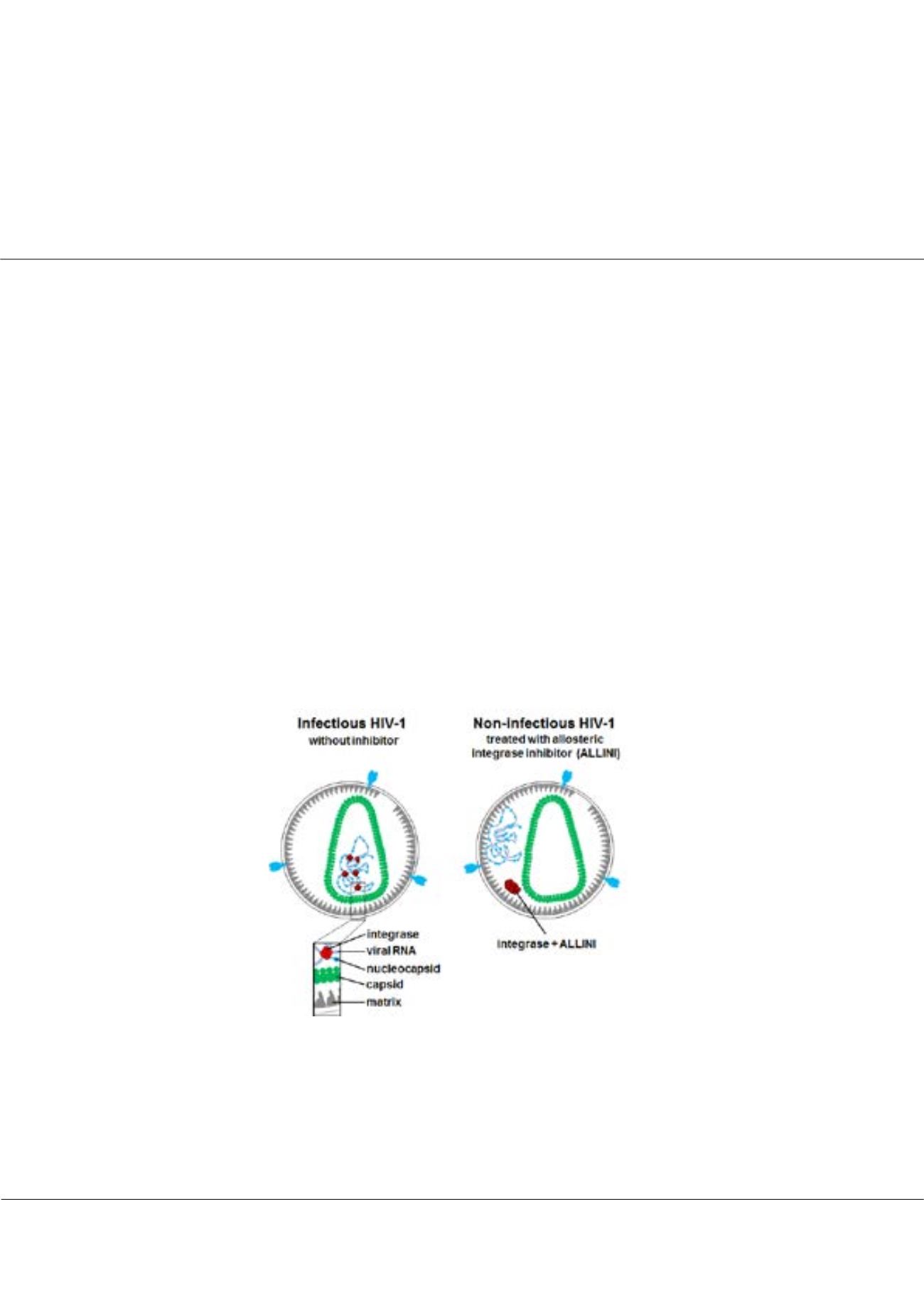
Figure 1:
The schematic to show that HIV-1 integrase has a second, non-catalytic function in HIV-1 biology as it binds the
viral RNA genome to promote particle maturation. ALLINI treatments mislocalize ribonucleoprotein complexes outside of the
protective capsid core.
Mamuka Kvaratskhelia, J Biotechnol Biomater 2018, Volume 8
DOI: 10.4172/2155-952X-C2-091
















