
Page 54
conferenceseries
.com
Volume 8
Journal of Biotechnology & Biomaterials
ISSN: 2155-952X
Biotech Congress 2018 & Enzymology 2018
March 05-07, 2018
JOINT EVENT
20
th
Global Congress on
Biotechnology
3
rd
International Conference on
Enzymology and Molecular Biology
&
March 05-07, 2018 London, UK
Stability and function of a thermophilic cytochrome c'
Sotaro Fujii
and
Yoshihiro Sambongi
Hiroshima University, Japan
C
ytochromes c' are classified as heme proteins found in restricted Gram-negative bacteria. They usually form a homo dimeric
structure, and the single subunit typically consists of four helix bundle. Biochemical analysis showed that they can bind diatomic
gasses such as NO or CO, but not O
2
. Recently we purified cytochrome c' from thermophilic
Hydrogenophilus thermoluteolus
, and
named it PHCP.
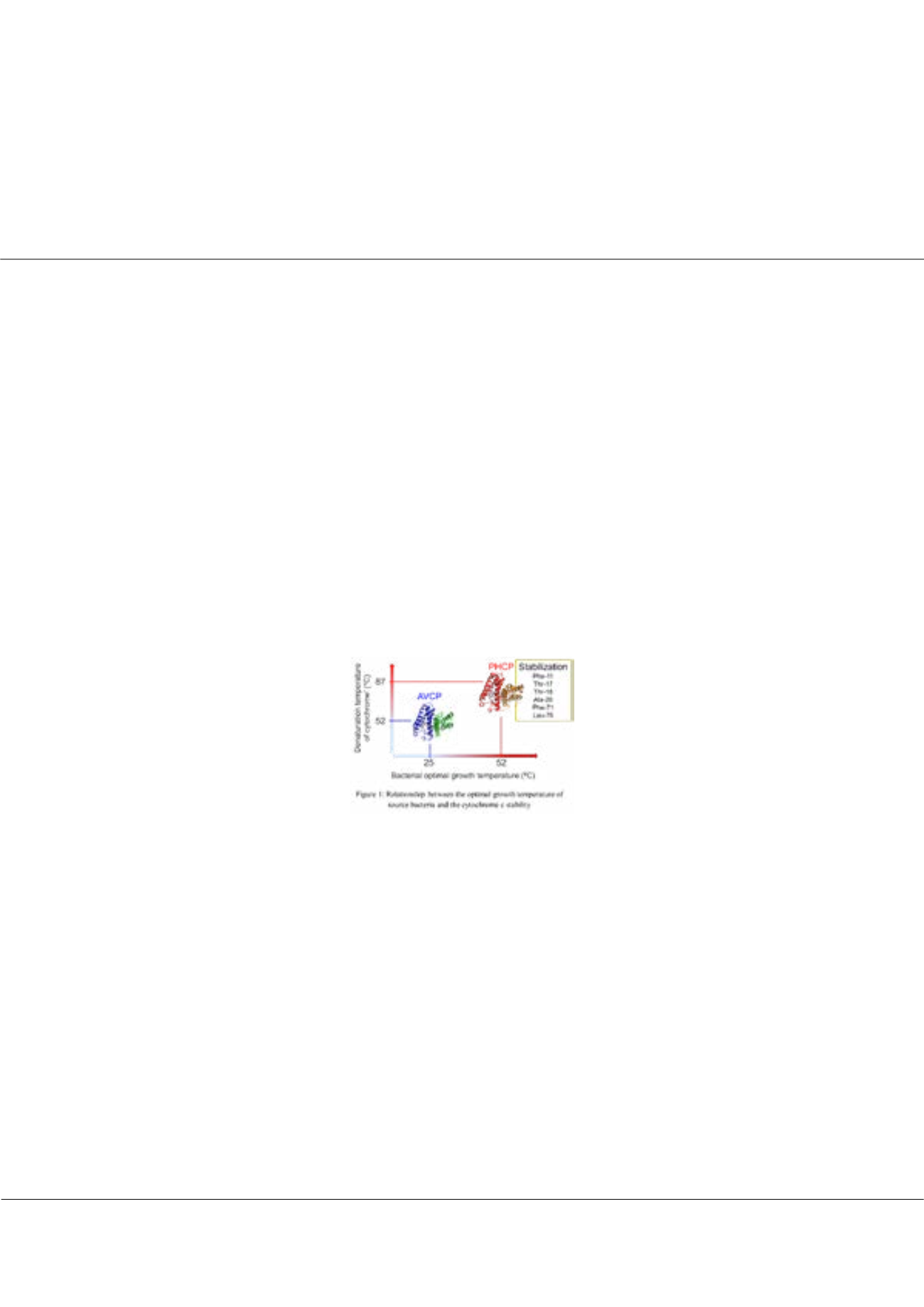
H. thermoluteolus
grows optimally at 52°C, indicating that PHCP is more stable than homologous proteins from
mesophiles. In this study, we compared stability and function of PHCP with its mesophilic homologue,
Allochromatium vinosum
cytochrome c' (AVCP) having 55 % amino acid sequence identity. In order to check the stability, we measured the circular dichroism
spectra with increasing temperature. The denaturation temperature of PHCP was 87°C, which was higher than that of AVCP
(52°C). The X-ray structure comparison between PHCP and AVCP revealed that the stability difference was due to the heme-related
interactions and subunit-subunit interactions, which was also proofed by mutagenesis study. These results indicated that PHCP
advantageously retains the native structure at high temperature. The PHCP X-ray structure further revealed a ligand binding channel
and a penta-coordinated heme, as observed in the AVCP protein, indicating PHCP could bind diatomic gasses at high temperature.
Thus, we measured the gas binding affinity of PHCP and AVCP using absorption spectra. The association constant (Ka) of PHCP
with CO was 3 times lower than that of AVCP at 25°C, and PHCP could maintain normal spectral changes up to 60°C. In AVCP, such
spectral changes with CO could not to be detected at 60°C, because of denaturation of AVCP. In conclusion, PHCP has a structure
fulfilling the requirement for both gas-binding function and thermal stability. This stable cytochrome c' will become a model for
protein engineering field.
Recent Publications
1.
Fujii S, Masanari M, Inoue H, Yamanaka M, Wakai S, Nishihara H, Sambongi Y (2013) High thermal stability and unique
trimer formation of cytochrome c' from thermophilic
Hydrogenophilus thermoluteolus
. Biosci Biotechnol Biochem 77:1677-
1681.
2.
Fujii S, Masanari M, Yamanaka M, Wakai S, Sambongi Y (2014) High stability of apo-cytochrome c' from thermophilic
Hydrogenophilus thermoluteolus. Biosci Biotechnol Biochem 78:1191-1194.
3.
Kato Y, Fujii S, Kuribayashi TA, Masanari M, Sambongi Y (2015) Thermal stability of cytochrome c' from mesophilic
Shewanella amazonensis. Biosci Biotechnol Biochem. 80: 2365-2370.
4.
Fujii S, Oki H, Kawahara K, Yamane D, Yamanaka M, Maruno T, Kobayashi Y, Masanari M, Wakai S, Nishihara H, Ohkubo
T, Sambongi Y (2017) Structural and functional insights into thermally stable cytochrome c' from a thermophile. Protein
Sci. 26: 737-748.
Biography
Sotaro Fujii is working on the stability, structure, and function of proteins that are important for microbial energy metabolism. A characteristic aspect of his research
activity is comparison of the homologous proteins isolated from microorganisms living in extreme environments in which humans cannot live and those isolated
from ‘normal’ environments.
sofuji@hiroshima-u.ac.jpSotaro Fujii, J Biotechnol Biomater 2018, Volume 8
DOI: 10.4172/2155-952X-C2-091
















