
Page 53
conferenceseries
.com
Volume 8
Journal of Biotechnology & Biomaterials
ISSN: 2155-952X
Biotech Congress 2018 & Enzymology 2018
March 05-07, 2018
JOINT EVENT
20
th
Global Congress on
Biotechnology
3
rd
International Conference on
Enzymology and Molecular Biology
&
March 05-07, 2018 London, UK
ProxiMAX randomization: Precision protein engineering
Anna V Hine
Aston University, UK
P
roxiMAX randomization is the technology that lies behind Isogenica’s Colibra™ offering. It is a defined saturation mutagenesis
process that delivers precision control of both identity and relative ratio of amino acids at specified locations within a protein/
antibody library. Thus unwanted amino acids such as cysteine and methionine can be eliminated from libraries because no constraints
are imposed by the genetic code. Moreover, the process is non-degenerate, which means that encoding DNA libraries are as small as is
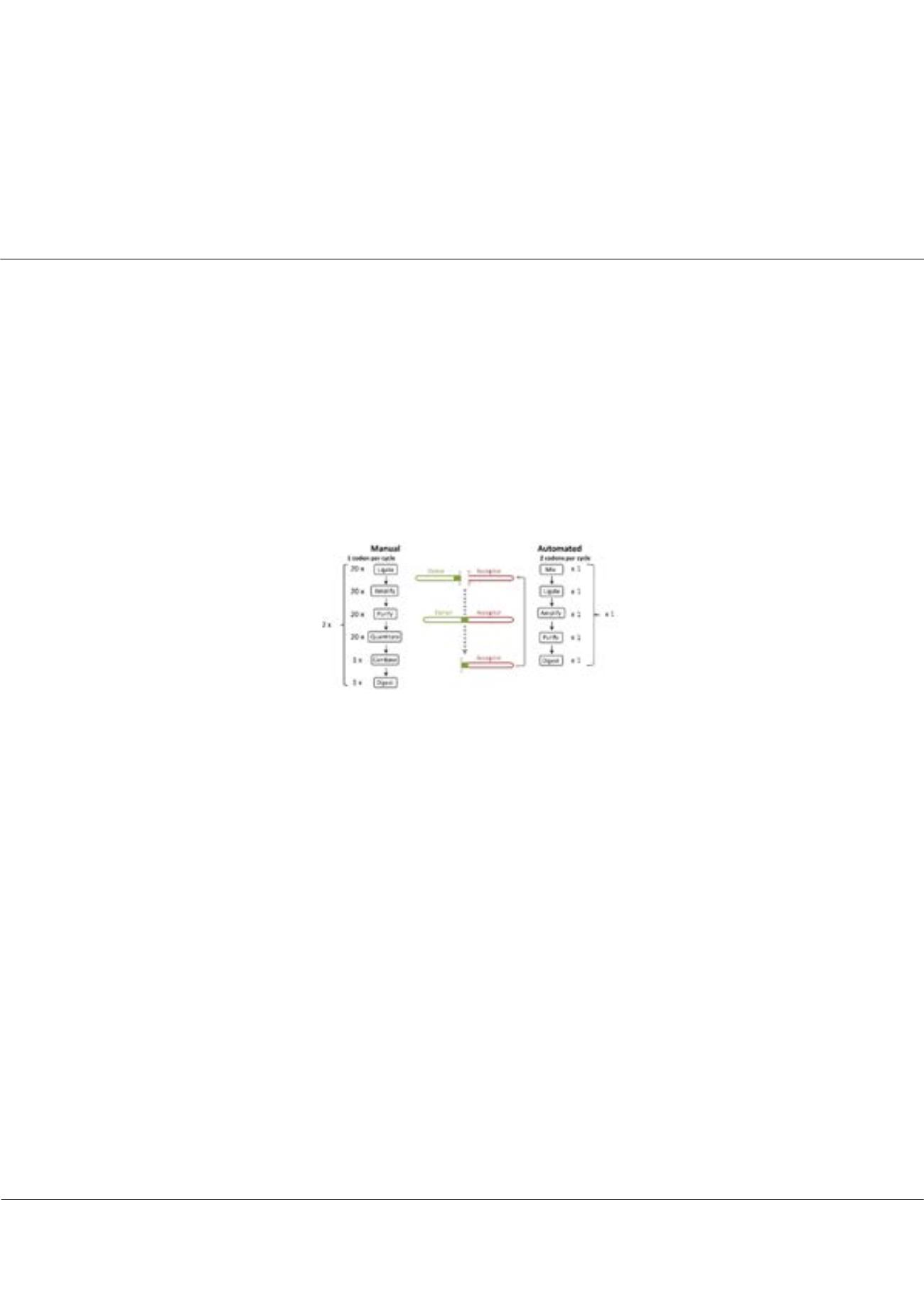
physically possible. ProxiMAX relies on a process of saturation cycling comprising ligation, amplification and digestion for each cycle
and is the science behind the commercial Colibra™ technology. Currently focused on antibody libraries but with achieved diversities
of >99% (6 & 11 saturated codons) and the potential to generate libraries of up to 10
14
components, we contest that ProxiMAX
randomization is a vital tool in engineering any protein library of the highest quality. This presentation will examine the development
of the ProxiMAX process and give examples of libraries created to date.
Figure 1:
Overview of the ProxiMAX
Recent Publications
1.
Ferreira Amaral MM, Frigotto L and Hine A V (2017) Beyond the natural proteome: nondegenerate saturation mutagenesis
- methodologies and advantages. Meth. Enyzmol. 585:111-133.
2.
Frigotto L, Smith M E, Brankin C, Sedani A, Cooper S E, Kanwar N, Evans D, Svobodova S, Baar C, Glanville J, Ullman C G
and Hine A V (2015) Codon-precise, synthetic, antibody fragment libraries built using automated hexamer codon additions
and validated through next generation sequencing. Antibodies 4:88-102.
3.
Chimonides G F, Behrendt J M, Chundoo E, Bland C, Hine A V, Devitt A, Nagel D A and Sutherland A J (2014) Cellular
uptake of ribonuclease A functionalised core–shell silica microspheres. J Mater Chem B, 2:7307-7315.
4.
Nagel D, Behrendt J M, Chimonides G F, Torr E E, Devitt A, Sutherland A J and Hine A V (2014) Polymeric microspheres
as protein transduction reagents. Mol. Cell Proteomics, 13:1543-1551.
5.
Ashraf M, Frigotto L, Smith M E, Patel S, Hughes M D, Poole A J, Hebaishi H R M, Ullman C G and Hine A V (2013)
ProxiMAX randomisation: a new technology for non-degenerate saturation mutagenesis of contiguous codons. Biochem.
Soc. Trans. 41:1189-1194.
Biography
Anna V Hine studied at the University of Manchester (UK) and Harvard Medical School. She is a Reader and Associate Dean Enterprise at Aston University (UK).
In March 2013, she was named BBSRC Commercial Innovator of the Year 2013, for her work in transferring ProxiMAX randomization into SME Isogenica Ltd. She
is a Molecular Biologist by training, she relishes interdisciplinary work.
a.v.hine@aston.ac.ukAnna V Hine, J Biotechnol Biomater 2018, Volume 8
DOI: 10.4172/2155-952X-C2-091
















