
Page 124
Notes:
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Stephen M Soisson, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100
Structural basis for the cooperative allosteric activation of the free fatty acid receptor GPR40
Stephen M Soisson
Merck Research Laboratories, USA
C
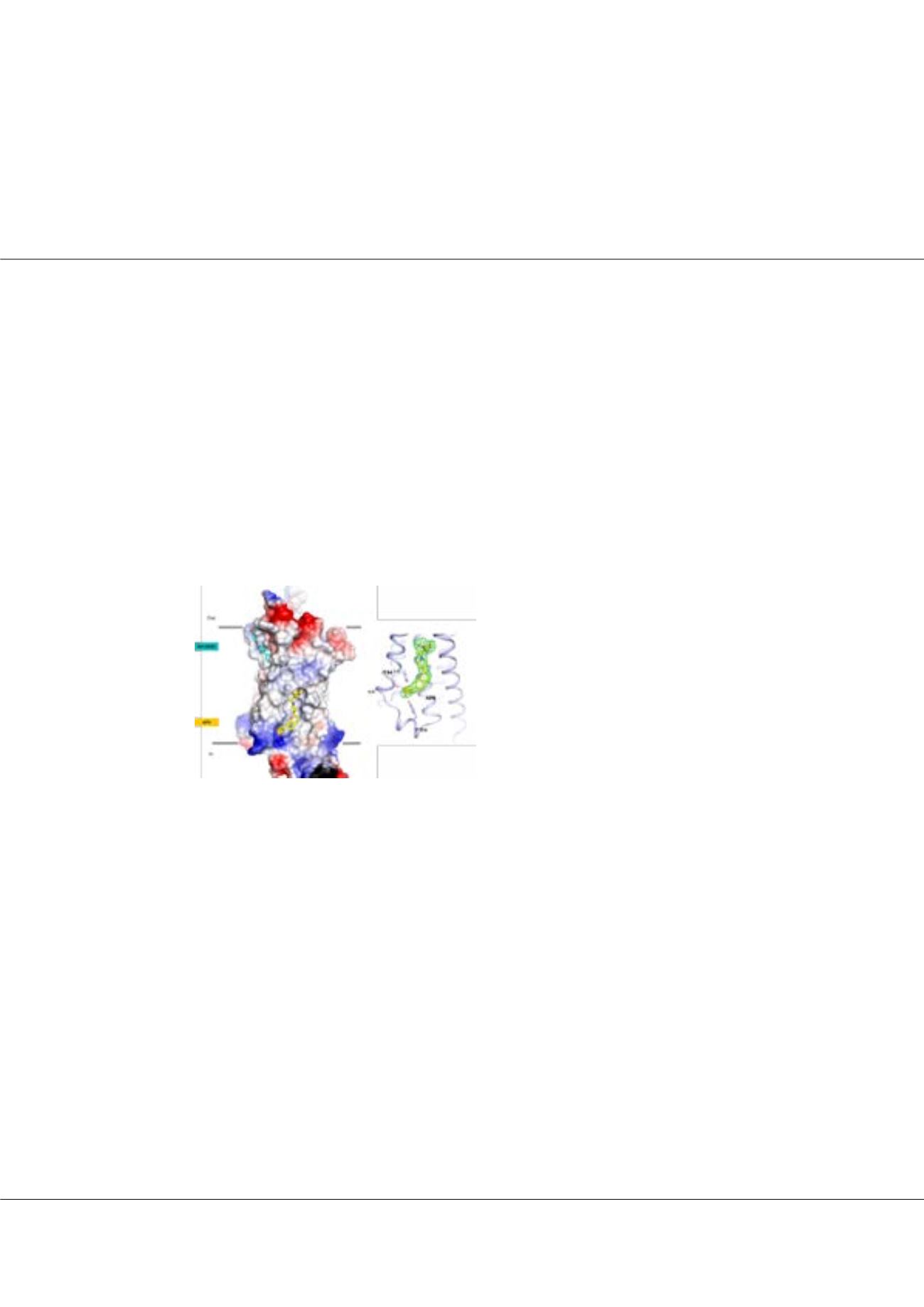
linical studies indicate that partial agonists of the G-protein-coupled, free fatty acid receptor GPR40 enhance glucose-
dependent insulin secretion and represent a potential mechanism for the treatment of type 2 diabetes mellitus. Recently
identified, full allosteric agonists (AgoPAMs) of GPR40 bind to a site distinct from partial agonists and can provide additional
efficacy. Our recent studies have led to a 3.2-Å crystal structure of human GPR40 (hGPR40) in complex with both the
partial agonist MK-8666 and an AgoPAM. Surprisingly, the structure reveals a novel lipid-facing AgoPAM-binding pocket
outside the transmembrane helical bundle. Comparison with an additional 2.2-Å structure of the hGPR40–MK-8666 binary
complex reveals an induced-fit conformational coupling between the partial agonist and AgoPAM binding sites, involving
rearrangements of the transmembrane helices 4 and 5 (TM4 and TM5). These structural rearrangements, along with AgoPAM
binding, appear to trigger the transition of intracellular loop 2 (ICL2) into a short helix. These conformational changes likely
prime GPR40 to a more active-like state and explain the binding cooperativity between these ligands.
Biography
Stephen M Soisson, PhD is a Director of Biochemical Engineering and Structure at Merck Research Laboratories in West Point, Pennsylvania (USA). With 25+
years of structural biology experience, he has focused research on elucidating the structural aspects of biological regulatory mechanisms, and applying these
insights in the area of structure-based drug design. He has served on the scientific advisory boards of the Structural Genomics Consortium, and the GPCR
Consortium.
stephen_soisson@merck.comFigure1:
Structural basis for the cooperative
allosteric activation of the free fatty acid receptor
GPR40


















