
Page 122
Notes:
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Guo-Ping Zhou, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100
The NMR studies of the interaction between polysialic acid (polySA) and the PSTD peptide in ST8Sia
IV polysialytranseferase
Guo-Ping Zhou
Gordon Life Science Institute, USA
T
he α 2,8-sialyltransferase (ST8Sia) family consists of 6 sia-lytranseferases, which are related to forms of polysialic acid chains
(PSA) on neural cell adhesion molecule (NCAM) and NCAM polysialylation, and have important effects on formation
of sialic acid storage diseases, neural system diseases and invasive cancers. It has been known that synthesis of PSA chains is
catalyzed by two polysialyltransferases, ST8Sia II (STX) and ST8Sia IV (PST). In addition, a polybasic motif of 32 amino acids
in both ST8Sia II and ST8Sia IV has been designated as “polysialytransferase domain” (PSTD), which is essential for NCAM
polysialylation. In this study, we have determined the 3D structure of the PSTD peptide containing 22 amino acids (22AA) in
ST8Sia IV using NMR spectroscopy. This NMR-based model displays that the PSTD domain consists of an α-helical segment,
two unstructured domains in both N- and C-terminus, and two three-residue-loops near the C-terminus of the peptide. Our
overlaid 2D 1H-15N-HSQC spectra of the 22AA-PSTD peptide show that the amide proton chemical shifts of some amino
acids such as I260, I261, H262, R265, L269 and K272 have been changed after polySA was mixed with the PSTD peptide. In
addition, the peak intensity of A263, V264, R265, Y267, L269 and K272 were also decreased after adding polySA. However,
there is no any change in both chemical shift and the amide proton peak intensity for all other residues located on outside of
the helix. Above NMR results indicate a weak interaction exists between the helix of the PSTD and the PolySA, which may play
a vital role in modulating biosynthesis of polySA chain and NCAM polysialylation.
Biography
Guo-Ping Zhou is currently a Distinguished Professor of Gordon Life Science Institute, USA. He is also an Adjunct Professor of several academics in the United
States and China. He received his PhD in Biophysics from University of California at Davis, and completed his Postdoctoral training at Stanford University
and Harvard University, respectively. He has determined the 3D NMR structures of some important proteins, protein-DNA complexes, and super lipids. He has
successfully introduced the elegant wenxiang diagrams to elucidate the biological mechanisms of protein-protein/ligands interactions observed by NMR. Meanwhile,
he has also published many papers in bioinformatics, and edited some special issues on structural biology for several influential scientific journals.
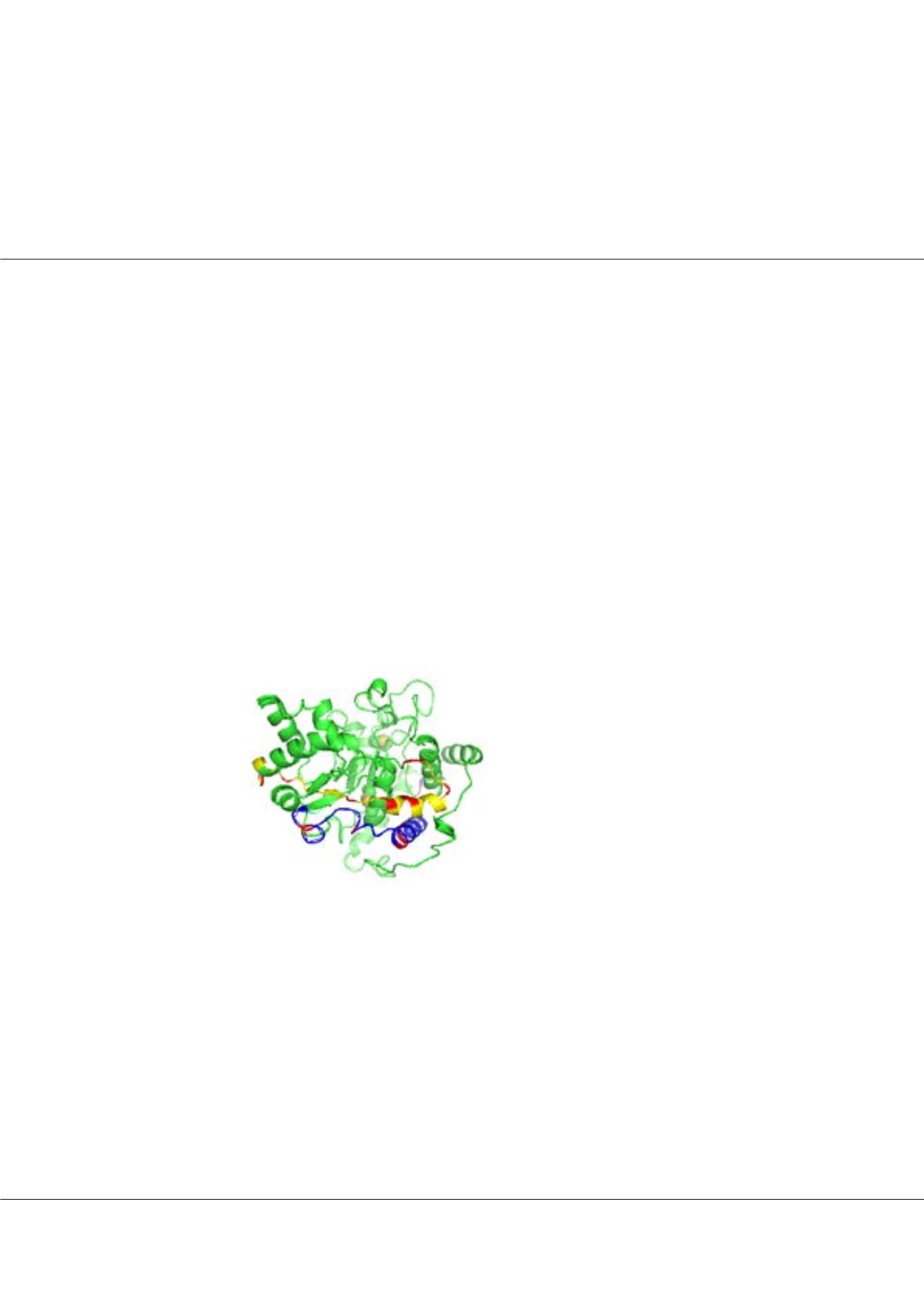
gpzhou@gordonlifescience.orgFigure1:
The 3D models of ST8Sia IV.
The PSTD domain is shown by yellow.
All basic residues in PSTD and PBR
motifs are shown by red.


















