
Page 38
conferenceseries
.com
Volume 8, Issue 6 (Suppl)
J Bioremediat Biodegrad, an open access journal
ISSN:2155-6199
Biopolymers & Bioplastics 2017
October 19-20, 2017
October 19-20, 2017 San Francisco, USA
7
th
International Conference and Exhibition on
Biopolymers and Bioplastics
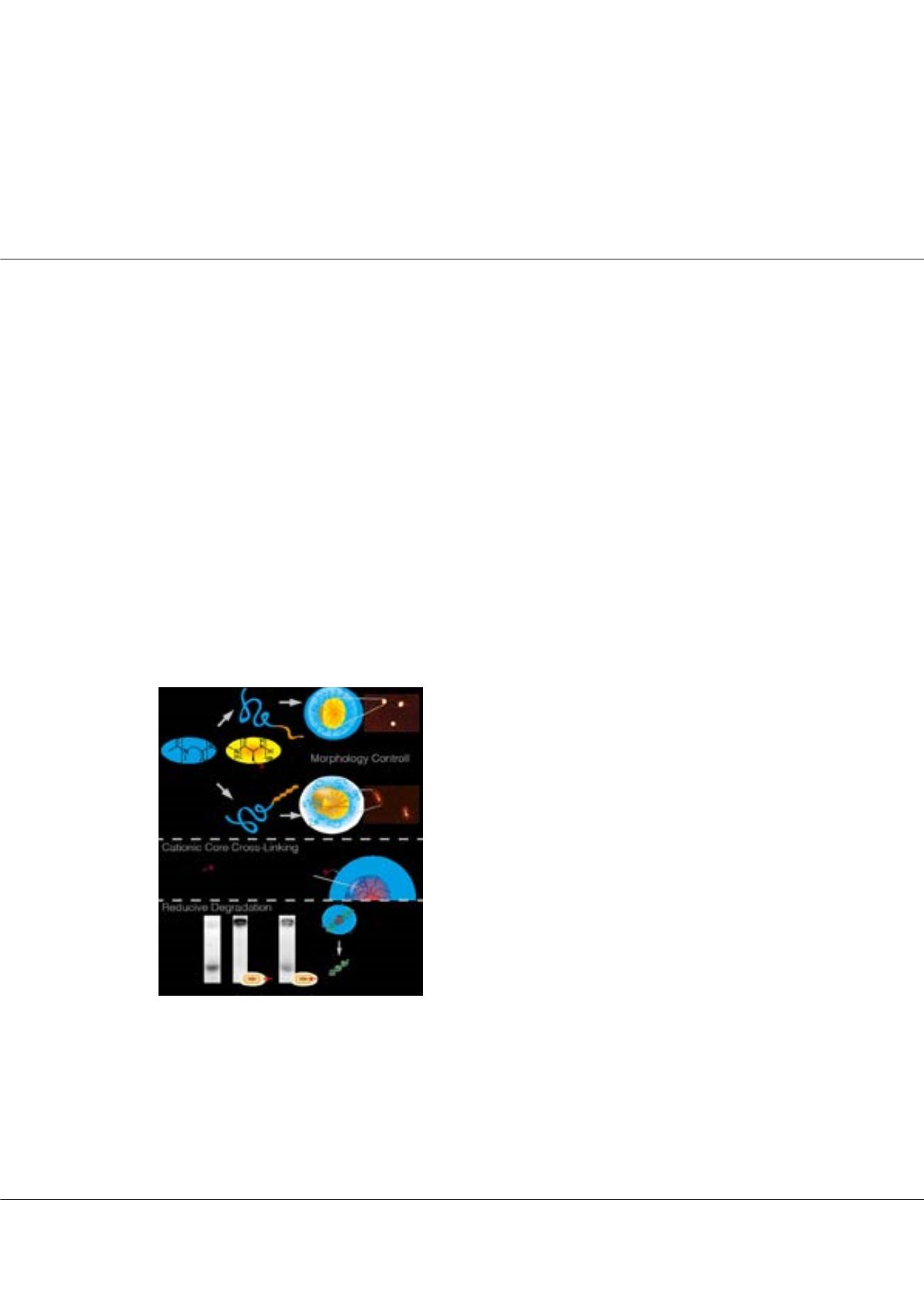
Secondary structure-driven self-assembly of reactive polypept(o)ides: Controlling size, shape und function
of core-crosslinked nanostructures
Olga Schäfer
Johannes Gutenberg University Mainz, Germany
T
he rational development of nano-sized delivery systems remains a challenge in the materials as well as bio-medical sciences,
especiallywhen independent control over size, shape and functionality of the carrier is desired. Herein, we report onnanostructures
derived from amphiphilic block copolypept(o)ides, based entirely on endogenous amino acids. Controlled self-assembly allows for
a strategy to adjust core polarity separately from particle preparation in a bio-reversible fashion. Additionally, the peptide-inherent
process of secondary structure-directed self-assembly allows for morphology control of core cross-linked nanostructures from the
same polymer precursor, offering a simple yet powerful approach to versatile peptide-based nanoparticles for delivery of various
therapeutic relevant agens such as chemotherapeutic drugs or siRNA. A recently developed S-ethylsulfonyl protective group enables
controlled polymerization of α-amino acid N-carboxyanhydrides (NCAs), directs self-assemblyin water and allows core-crosslinking
by a fast chemoselective reaction into bio-reversible disulfides. The hydrophobic block is further able to form β-sheets,leading to
unidirectional aggregation in aqueous solution and ultimately to worm-like particles, or, after suppression of secondary structures, to
spherical micelles. Independent from the morphology, the crosslinker dictates the functionality of the nanoparticle core and enables
the introduction of cationic moieties for siRNA complexation. Given the bioreversible nature of disulfide bonds, they respond to
differences in reduction potential and hence provide stability in extracellular medium, while they are cleaved inside the cell and
release the cargo.
Biography
Olga Schäfer studied Biomedical Chemistry at the Johannes Gutenberg University Mainz and obtained her graduate degree in 2014. After her Diploma thesis on
Sethylthiosulfonyl- L-cysteine in peptide synthesis she started her PhD on the implementation of reactive block copolypept(o)ides for biomedical applications in the
junior research group of Matthias Barz. The developed multifunctional polymers are applied in the shape controlled self-assembly of cross-linked nanostructures
for delivery of therapeutic cargos such as chemotherapeutic drugs and nucleic acids.
olga.schaefer@uni-mainz.deOlga Schäfer, J Bioremediat Biodegrad 2017, 8:6 (Suppl)
DOI: 10.4172/2155-6199-C1-011
Figure 1: Self-assembly of amphiphilic PSar-b-PCys(SO
2
Et) block
copolypept(o)ides yields spherical or rod-like nanoparticles, depending
on the secondary structure of the polymer. After self assembly the
core functionality is introduced by disulfide core-crosslinking, which
proves to be bioreversible.
















