
Page 62
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
A structure complex of a bacterial effort and an interacting protein: Insights into the transfer of
virulence effector by pathogenic bacteria
Jianyong Li, Han Qian
and
Binyu Zhao
Virginia Tech, USA
B
acterial effectors are proteins secreted by pathogenic bacteria into host cells through a type 3 or 4 secretion system. These
bacterial effectors may help the pathogens to invade host cells and/or suppress its immune system; thereby promoting
their infection, survival and reproduction. Effector proteins are primarily responsible for the pathogenicity of a given bacterial
pathogen; therefore, learning the specific mechanism but which their effectors enter into host cells may provide insights for
disease prevention. Bacterial Type 3 Secretion System (T3SS) has been extensively studied. It is a needle-like structure made by
a number of structural proteins, which is responsible for transfer of protein effector to host cells. The needle tip is ~ 3 nm, which
is smaller than the required dimension for most bacterial effectors. Despite some better understanding of the T3SS structure,
how their bacterial effectors gain entry to host cells remains speculative. Using a T3SS-dependant effector as a model from
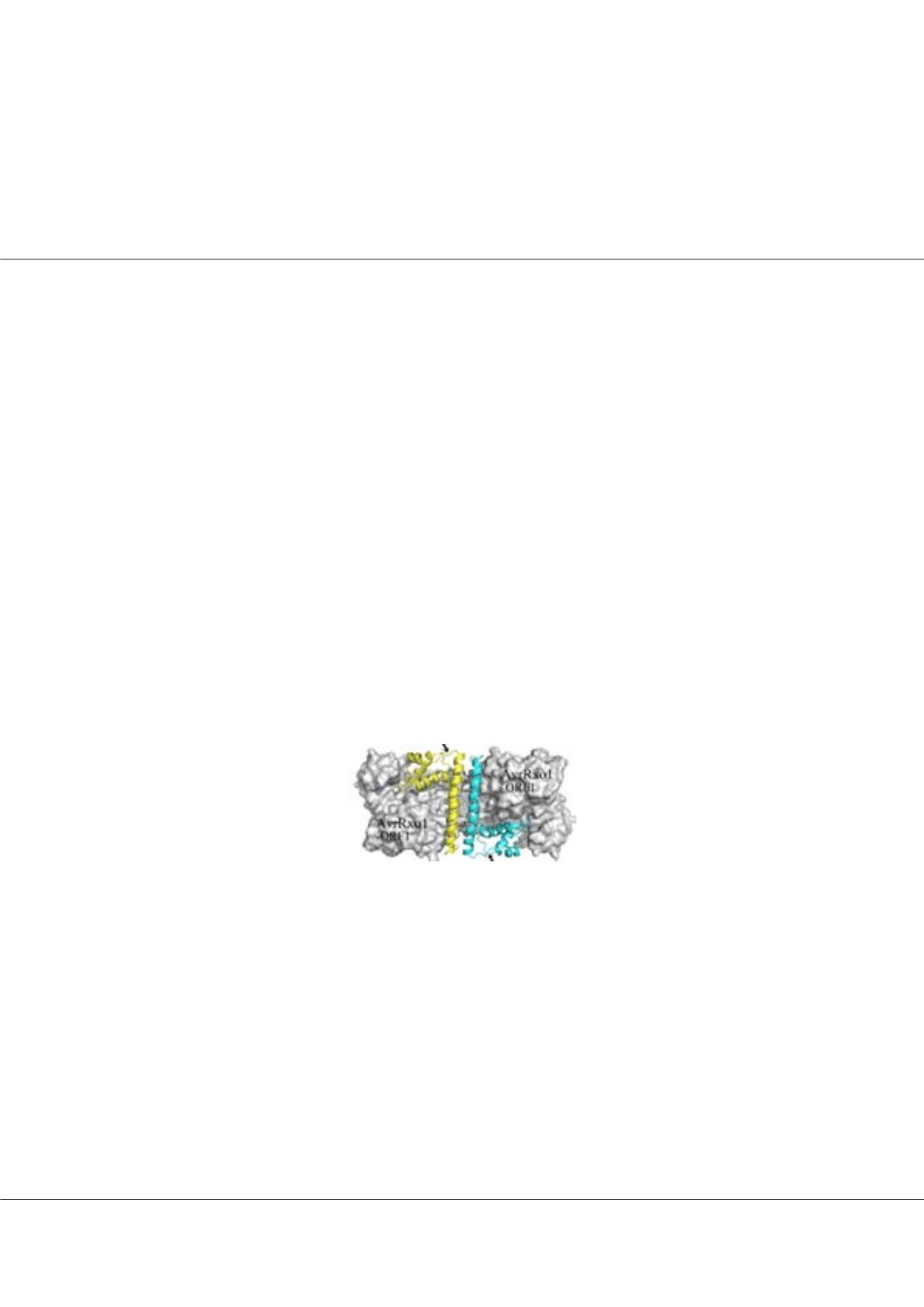
Xanthomonas oryzae
(a bacterium causing serious disease to some essential plants), we determined the structures of an effector
protein in complex with a chaperone-like protein. In the genome of
Xanthomonas oryzae
, the coding sequence of effector
protein is adjacent to that coding the chaperone-like protein. Our structural analysis indicates that the effector-chaperon
complex crystallized as tetramers (1.64 Å resolution). The monomer of the protein effector contains a T4 polynucleotide kinase
domain, while the monomer of the chaperon includes a novel kinase binding domain. Our data suggest that the chaperone
protein interacts with the protein effector in a manner that helps to stabilize the protein effector and prevents the virulence
effect of protein effort from harming the bacteria before being transferred to host cells. Currently, efforts are being made to
understand the precise roles the chaperon protein plays during the transfer of protein effector to host cells.
Biography
Jianyong Li is a Biochemistry Professor at Virginia Tech. He has extensive experience in protein-related studies, including protein expression and purification,
protein functional determination and protein structure and function relationships. Particularly worth to mention is the functional establishment of some unique yellow
genes in insects and structural and function relationship of enzymes involved in kynurenate synthesis in mammals.
lij@vt.eduJianyong Li et al., J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100


















