
Page 61
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Structural analysis of vascular endothelial growth factor receptors reveals drug-targetable allosteric
sites regulating angiogenesis
Kurt Ballmer-Hofer, Sandra Markovic-Mueller, Edward Stuttfeld
and
Dragana Avramovic
Paul Scherrer Institut, Switzerland
V
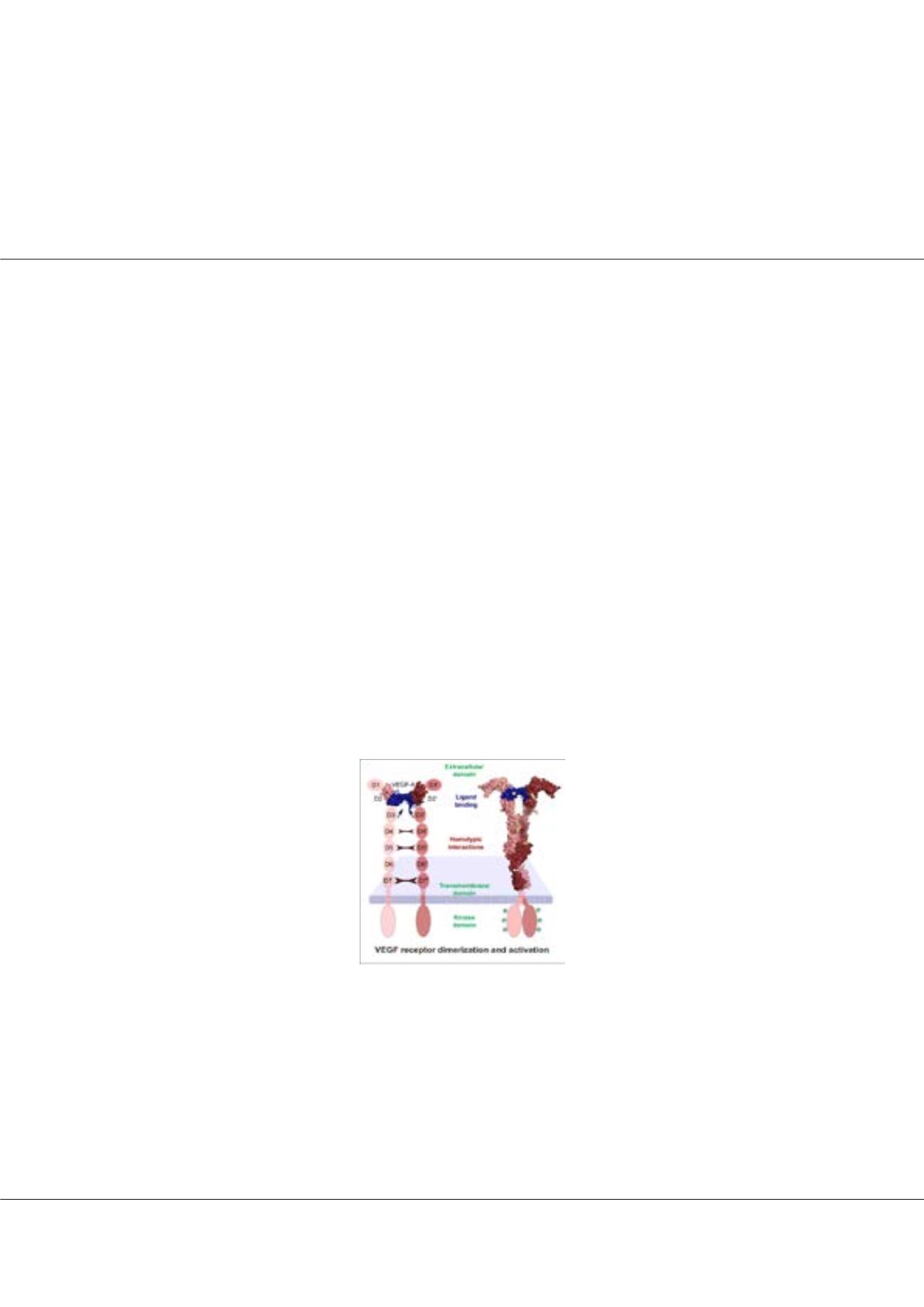
ascular Endothelial Growth Factors (VEGFs) regulate blood and lymph vessel development upon activation of three
receptor tyrosine kinases (RTKs), VEGFR-1, -2, and -3. Partial structures of VEGFR/VEGF complexes based on single
particle electron microscopy, small angle X-ray scattering, and X-ray crystallography revealed the location of VEGF binding
and the spatial arrangement of individual receptor subdomains. Here we describe the structure of the full-length VEGFR-1
extracellular domain (ECD) in complex with VEGF-A at 4 Å resolution. We combined X-ray crystallography, single particle
electron microscopy, and molecular modeling for structure determination and validation. The structure reveals the molecular
details of ligand-induced receptor dimerization, in particular of homotypic receptor interactions in Ig-domains 4, 5, and 7.
Functional analyses of ligand binding and receptor activation confirm the relevance of these homotypic contacts for receptor
activation and identify them as allosteric regulatory sites of VEGFR-1. Based on our structural data we also investigated the
function of Ig-domains 4, 5 and 7 in VEGFR-2, the primary receptor driving angiogenesis and vasculogenesis in response
to VEGF administration. The basic domain structure of VEGFR-2 is very similar to VEGFR-1, the ECD of both receptors
consists of 7 Ig-domains, D1-D7. Mutagenesis studies based on the VEGFR-1structure confirmed that Ig-domains 4 and 7
fulfill an essential regulatory function in receptor activation and may thus represent putative targets for pharmacological
intervention. We isolated highly specific antibodies and DARPins (Designed Ankyrin Repeat Proteins) specific for domains
4 or 7. A subset of these reagents efficiently blocked receptor activation and inhibited VEGF-dependent signaling
in vitro
in
endothelial cell cultures. Most importantly, a domain 4-specific DARPin efficiently blocked vessel development also
in vivo
in
a mouse angiogenesis model. In this model endothelial cell spheroids were implanted in matrigel into mice, and cell growth
and vessel formation were monitored in the absence and presence of inhibitor. Our study thus revealed a novel approach for
therapeutic targeting of aberrant blood vessel development.
Biography
Kurt Ballmer-Hofer focused his research at PSI on the structural and functional analysis of receptor tyrosine kinases, in particular on Vascular Endothelial Growth
Factor Receptors, VEGFRs. In collaboration with partner labs his team solved the structures of VEGF ligands, the ligand binding domain of VEGFR-2, and -3, and
of the full-length extracellular domain of VEGFR-1 in complex with VEGF. The data of these studies led to the discovery of allosteric receptor regulatory sites in
subdomains 4, 5 and 7. Antibodies and DARPins specifically binding to these domains showed strong inhibition of receptor activation and downstream signaling
both
in vitro
and
in vivo
in angiogenesis model systems.
kurt.ballmer-hofer@unibas.chKurt Ballmer-Hofer et al., J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100


















