
Page 63
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Complex structure of mammalian cytochrome c–cytochrome c oxidase reveals a novel protein-protein
interaction mode
Kyoko Shinzawa-Itoh
Hyogo University, Japan
M
itochondrial cytochrome c oxidase (CcO) transfers electrons from cytochrome c (Cyt.c) to O2 to generate H2O, a process
coupled to proton pumping. To elucidate the mechanism of electron transfer, a crystal structure of the complex of CcO
and Cyt.c would be invaluable for mechanistic studies. Two-dimensional (2D) crystals of the mammalian Cyt.c–CcO complex
were prepared at higher pH (7.4–9.0) with both proteins in the oxidized state (Osuda et al, 2016), but these 2D crystals could
not provide us with a structure of sufficient resolution. We optimized 3D crystallization conditions for ferri-Cyt.c and oxidized
CcO at high pH and solved the X-ray structure of the complex at 2.0 Å resolution. The specific interaction between Cyt.c and
CcO is stabilized by only six electrostatic interactions between side chains within a small contact surface. From a theoretical
calculation based on the complex structure, we identified an electron transfer pathway from the heme c of Cyt.c to CuA of CcO
via
Lys-13 of Cyt.c. Between the two proteins are three water layers, one of which lies between the other two layers without
significant direct interaction with either protein. The inter-molecular span between Cyt.c and CcO is longer than those of other
complexes by more than 3.0 Å, and the contact surface area of Cyt.c and CcO is smaller than one-third the size of those of other
complexes. Cyt.c undergoes large structural fluctuations, using the interacting regions with CcO as a fulcrum. These features
of the protein–protein interaction at the docking interface represent the first known example of a new class of inter-protein
interaction, which we term “soft and specific”. This interaction is predicted to contribute to the rapid association/dissociation
of the Cyt.c–CcO complex, which facilitates the sequential supply of four electrons for the O2 reduction reaction.
Biography
Kyoko Shinzawa-Itoh, Associate Professor of University of Hyogo, grew up in Hiroshima Japan. She received her MS degree from Hiroshima University of Graduate
School of Integrated Arts and Sciences and her Ph D. in Pharmaceutical Sciences from Hiroshima University of Graduate School of Biomedical & Health Sciences.
She worked as assistant professor at the Department of Life Science, Himeji Institute of Technology 1988-2004 and at the Hyogo University of Graduate School
of Life Science 2004-2013. She is associate Professor of Picobiology Institute, Graduate School of Life Science University of Hyogo from 2013. She has studied
about mitochondrial respiratory complexes.
shinzawa@sci.u-hyogo.ac.jpKyoko Shinzawa-Itoh, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100
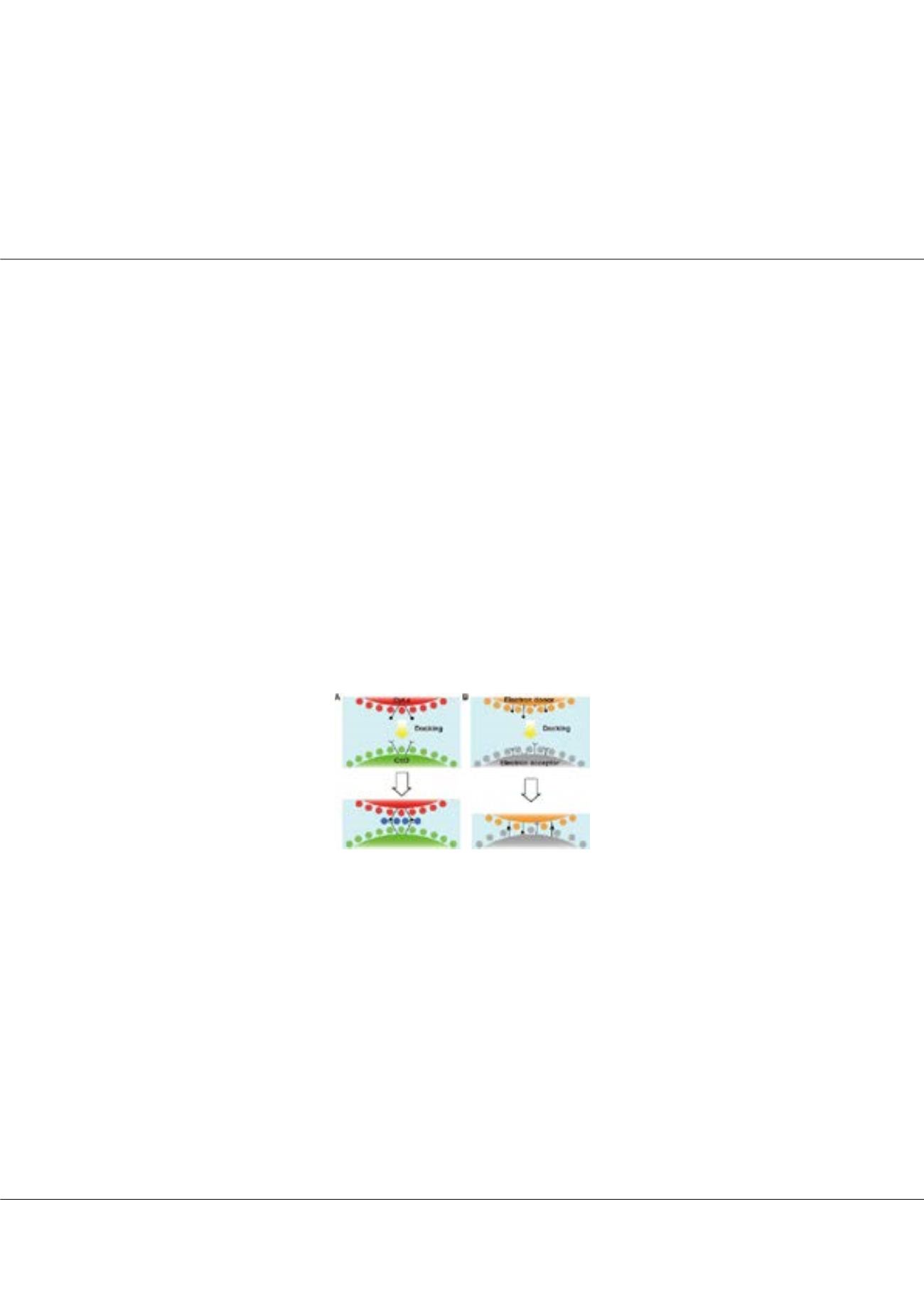
Figure1:
A In the Cyt.c–CcO complex system, water molecules on the surfaces of each protein
are preserved to form three layers upon docking, but each protein specifically interacts via the long
arms of side chains. B In other ET complex systems, electron donor and acceptor proteins form
an ET complex by excluding water molecules on the surface of each protein.


















