
Page 55
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Does the dynamics of their transmembrane domain qualify bitopic membrane proteins as substrates
for intramembrane proteolysis?
Christina Scharnagl
Technical University of Munich, Germany
I
ntegral membrane proteins facilitate communication between the inside of the cell and its exterior. Their transmembrane
domains (TMDs) support a diversity of biological functions and exhibit sequence-dependent conformational dynamics
on multiple size and time scales. Membrane proteins are notoriously difficult to study by experimental methods. Molecular
dynamics (MD) simulations provide a powerful tool of high spatial and temporal resolution that effectively complements
experimental methods. Here we focus on the conformational dynamics of the TMD of the amyloid precursor protein (APP).
APP is enzymatically hydrolyzed within its TMD by γ-secretase (GSEC), forming toxic Aβ peptides regarded as molecular cause
of Alzheimer's disease (AD). Besides APP, GSEC cleaves ~100 single-span membrane proteins within their TMDs, however
without obvious consensus sequence. Finding the link between the molecular architecture of the substrate TMDs and cleavage
is, therefore, of upmost importance. Because unfolding is obvious to expose the scissile bond, it seems plausible that the TMD
itself is optimized for local helix unwinding. However, this view was challenged by our experiments and MD simulations. Our
results suggest an alternative model where reaching a cleavage-competent state involves multiple conformational transitions of
the substrate/enzyme complex where global conformational plasticity of the substrate TMD is a key determinant. In a first step,
we compare the conformational flexibility of a large number of substrate and non-substrate TMDs, as well as TMDs carrying
missense mutations related to early onset AD. Knowing the key-dynamical motifs will help to identify new substrates and to
elucidate the physiological functions of the protease in the brain and other organs. This work is part of a collaborative research
program
(https://www.i-proteolysis.de/).
Biography
Christina Scharnagl has her expertise in molecular dynamic simulations of membrane proteins. Her work focuses on biophysical principles of the interdependence
of transmembrane helix dynamics, helix-helix binding, and helix-lipid interactions.
In silico
modelling and advanced computational analysis are closely connected
to experimental work in research collaborations in order to interpret and guide the experiments and to validate the simulations. The aim of the joint efforts is to
understand the impact of these phenomena on multiple biological processes, such as membrane fusion and intramembrane proteolysis.
christina.scharnagl@tum.deChristina Scharnagl, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100
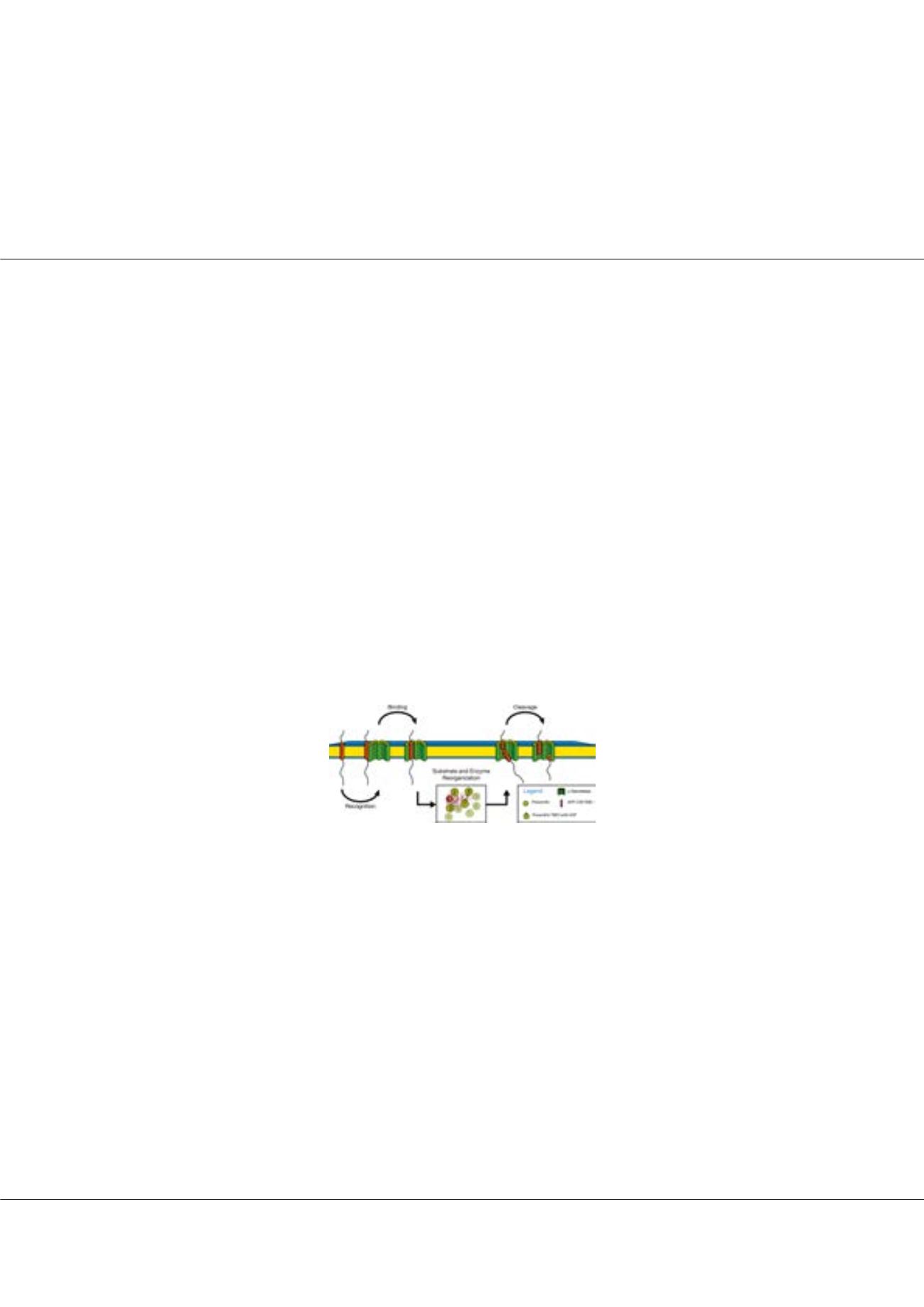
Figure1:
Substrate processing by γ-secretase. The intramembrane protease is a protein complex hydrolyzing substrates
within their trans-membrane domains. Transmembrane domain dynamics might be involved in recognition, binding and
reorganization steps funnelling the enzyme/substrate complex towards the conformation conducive for cleavage.


















