
Page 45
conferenceseries
.com
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18-20, 2017
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
Complex of malaria parasites and human proteins drive formation of cytoadherent assemblies at the
surface of infected red blood cells
John Vakonakis
University of Oxford, United Kingdom
H
uman red blood cells infected by the malaria parasite
Plasmodium falciparum
(iRBC) form dome-shaped ~120 nm-
diameter protrusions on their surface, known as ‘knobs’. Knobs provide essential presentation platforms for the parasite
cytoadherence receptor family P
f
EMP1, which binds ligands on endothelial cells of the blood vessel wall thereby immobilizing
iRBC in the microvasculature. The resulting obstruction of blood vessels and disruption of normal circulation causes
inflammation and tissue damage that can lead to coma and death. iRBC cytoadherence constitutes the primary mechanism
driving morbidity and mortality in
P. falciparum
infections, which account for over 90% of all malaria-related deaths. Despite
their importance in malaria pathology the molecular mechanisms underpinning knob formation remain poorly understood.
Here, I review recent progress in characterizing knob complexes formed between parasite and parasite-host proteins. Extensive
flexibility is common among parasite knob components, which necessitated an integrative approach to resolve these complexes.
I will focus on the development of novel
in silico
docking tools suitable for evaluating interactions between folded components
and highly charged, very long and flexible protein segments. Our work offers the first glimpse of a molecular model for knobs.
Biography
John Vakonakis has completed his PhD in Biochemistry at Texas A&M University, where he pioneered the structural analysis of bacterial circadian clock proteins.
His Postdoctoral work at the University of Oxford focused on the structural mechanisms underpinning cell adhesion and assembly of the extracellular matrix in
animals. He did breakthrough work on the molecular architecture of the centriole organelle during a second Postdoc at the Swiss Light Source, prior to starting his
own lab in Oxford Biochemistry. He has been a Marie Currie Fellow, Junior Research Fellow at Trinity College, Oxford, and a Wellcome Trust Research Fellow.
He is now Associate Professor in Structural Biology and Biophysics at the University of Oxford, and Fellow in Biochemistry at Lincoln College. Over the last six
years his research aims to understand how large molecular machines form in cells, such as the cytoadherence assemblies created upon
P. falciparum
-infection of
human erythrocytes.
ioannis.vakonakis@bioch.ox.ac.ukJohn Vakonakis, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100
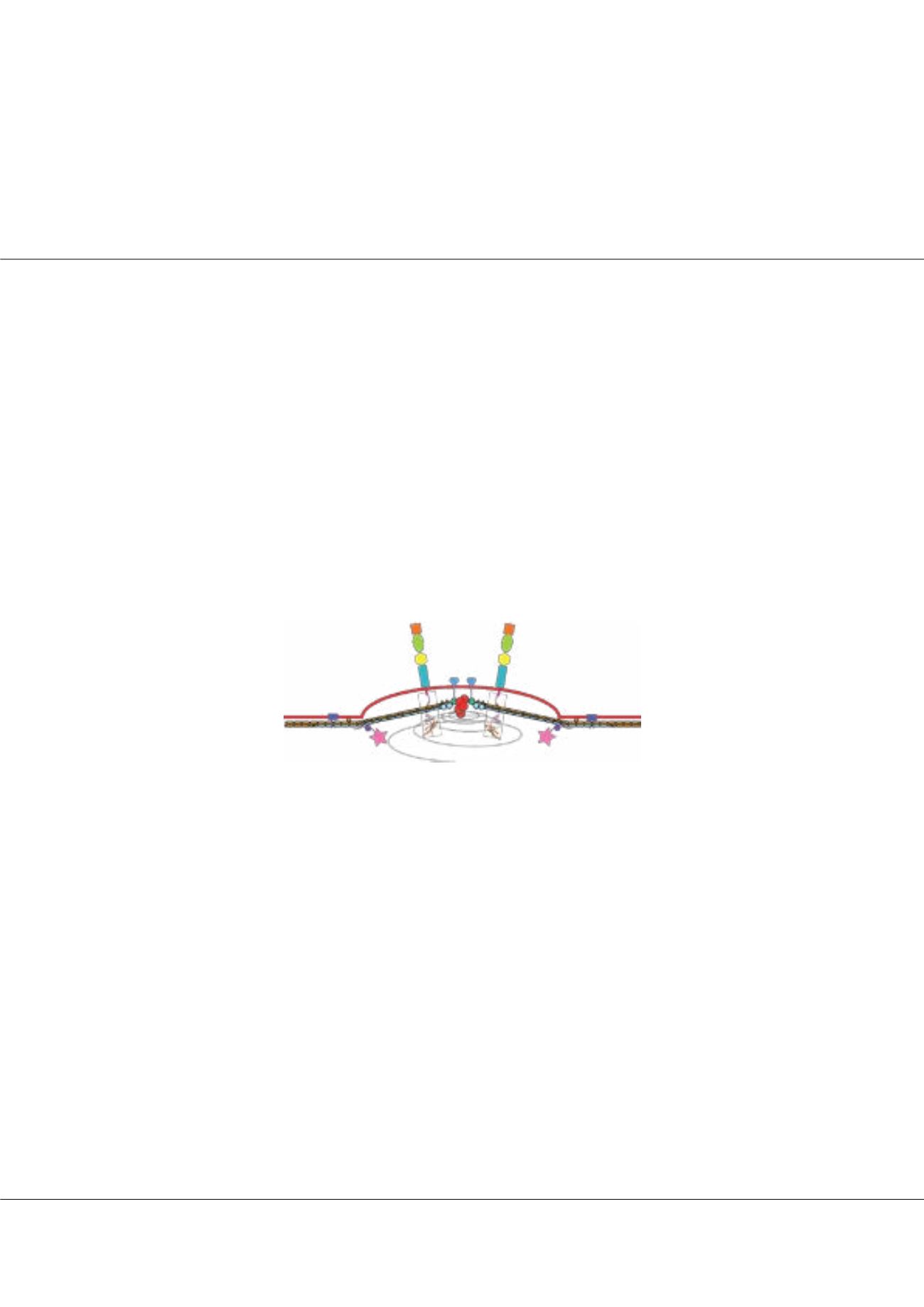
Figure1:
Model of a cytoadherent assembly (‘knob’) on the surface of P.
falciparum-infected red blood cell, depicting recently characterized protein
complexes that will be discussed here.


















