
Page 64
Notes:
conferenceseries
.com
Volume 5, Issue 3 (Suppl)
Mod Chem Appl, an open access journal
ISSN: 2329-6798
Global Chemistry 2017
September 04-06, 2017
September 04-06, 2017 | London, UK
5
th
Global Chemistry Congress
Synthesis and antioxidant activity of 2-mercaptothienopyrimidine-tethered-1,2,3-triazoles
Parvesh Singh
and
Nagaraju Kerru
University of Kwa-Zulu Natal, South Africa
T
hienopyrimidines are known to possess a wide-spectrum of medicinal activities including anti-TB, anti-cancer, anti-
bacterial, anti-fungal, anti-oxidant, and anti-diabetic activities. Similarly 1, 2, 3-triazole nucleus has been exploited in the
design of several medicinally and biologically active scaffolds including anti-oxidant compounds. Among different synthetic
approaches being used in the drug discovery, molecular hybridization (MH) is gaining popularity owing to its ability to combine
two or more bioactive scaffolds with different or novel mechanism of action. In view of the aforementioned significance of
thienopyrimidine and 1,2,3-triazoline scaffolds, it was thought worthwhile to couple these two pharmacophores in a single
molecular architecture to check the anti-oxidant effect of the resultant molecular hybrid. Accordingly, the desired molecular
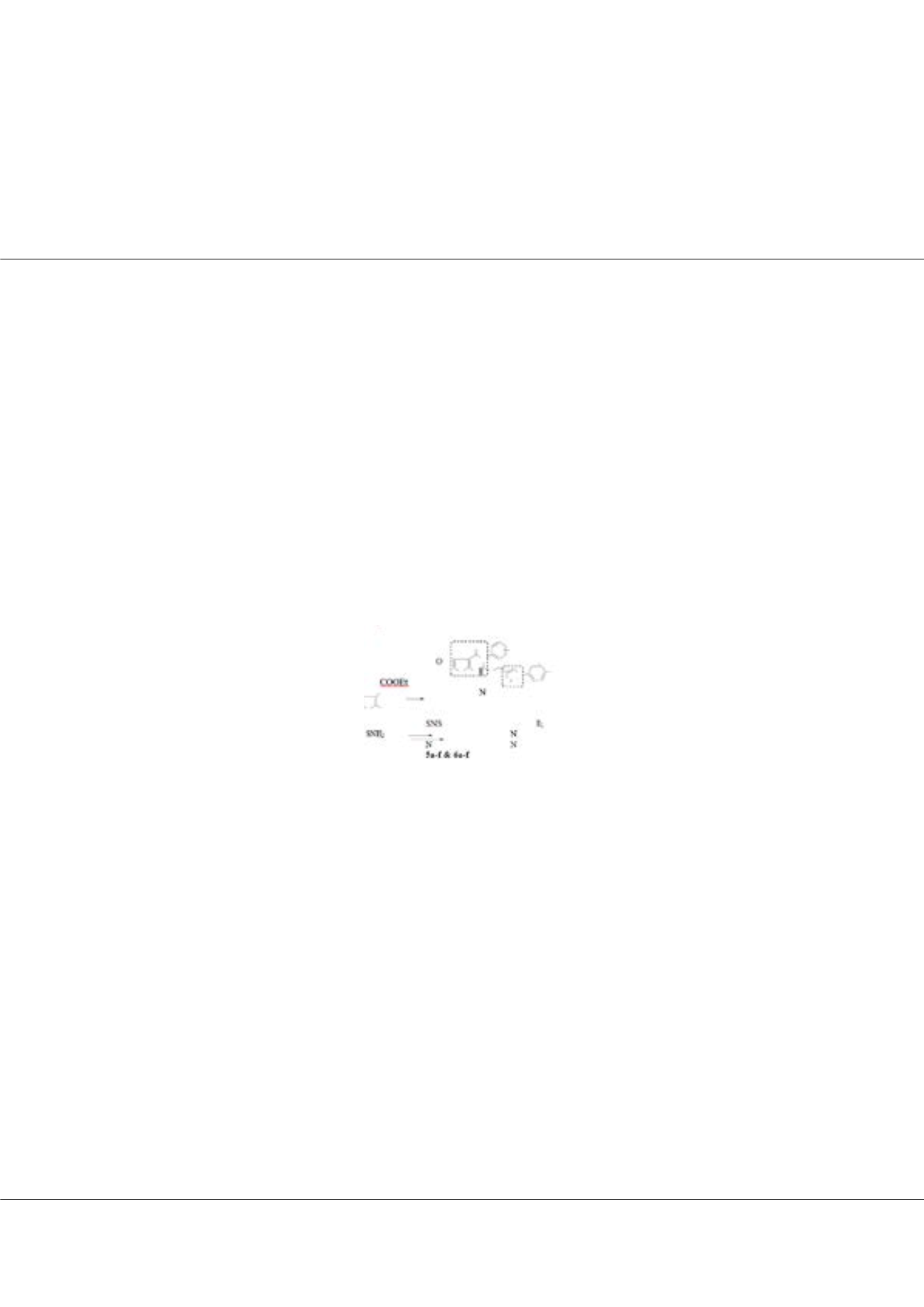
hybrids (5a-f and 6a-f) were prepared by copper catalyzed cycloaddition of alkynyl thienopyrimidine with various substituted
aryl azides in the presence of CuSO4 and sodium ascorbate in THF/H2O (1:1). The synthesized compounds screened for their
antioxidant activity using DPPH radical scavenging, NO and ABTS assays revealed several promising molecular conjugates
with excellent antioxidant activity, even better than the standard drug (acarbose). The structure activity relationship studies
further revealed the role of electron-donating (-CH3, Cl, I) groups in increasing the anti-oxidant activity of the compounds,
whereas an opposite effect was observed for the electron-withdrawing substituents.
Biography
Parvesh Singh received his PhD degree in Organic Chemistry from the Guru Nanak Dev University, India. Currently, he is working as a Senior Lecturer of Organic
Chemistry at University of KwaZulu Natal (South Africa). His research interests involve the synthesis, biological evaluation and molecular modeling of heterocyclic
scaffolds. He is primarily using hetero Diels-Alder methodology to synthesize heterocyclic rings of different sizes. He has published 60 research articles in peer-
reviewed journals of international repute including a book chapter and a book.
parveshdurban@gmail.comParvesh Singh et al., Mod Chem Appl 2017, 5:3(Suppl)
DOI: 10.4172/2329-6798-C1-006
















