
Page 62
Notes:
conferenceseries
.com
Volume 5, Issue 3 (Suppl)
Mod Chem Appl, an open access journal
ISSN: 2329-6798
Global Chemistry 2017
September 04-06, 2017
September 04-06, 2017 | London, UK
5
th
Global Chemistry Congress
Full structural elucidation of quinazoline-Schiff bases using 2D-NMR studies
Neha Manhas, Parvesh Singh
and
Neil A Koorbanally
University of KwaZulu-Natal, South Africa
Q
uinazoline nucleus is not only present in various therapeutic agents but also form an important structural component of
natural products. The prazosin (anti-hypertensive), methaqualone (hypnotic), metolazone (diuretic), raltitrexed (anti-
cancer) and (+)-febrifugine (anti-malarial) are few commonly used drugs that contains quinazoline ring as a core. Over the past
few decades, the synthesis of quinazoline derivatives has attracted considerable attention of the synthetic chemists worldwide
owing to its diverse medicinal activities including anticancer, anti-HIV, antibacterial, anti-inflammatory, anti-malarial, and
anti-tubercular activities. The most established preparative methodologies for quinazoline synthesis involve its use. On
the other hand, Schiff bases (>C=N), also known as imines or azomethines, are another widely explored intermediates in
heterocyclic synthesis with various biological/medicinal applications such as anticancer, anti-tubercular and anti-inflammatory
activities. Consequently, several research papers and review articles regarding the design and synthesis of new and promising
quinazolines or Schiff bases or both as their hybrids have been documented in international peer-reviewed literature. In view
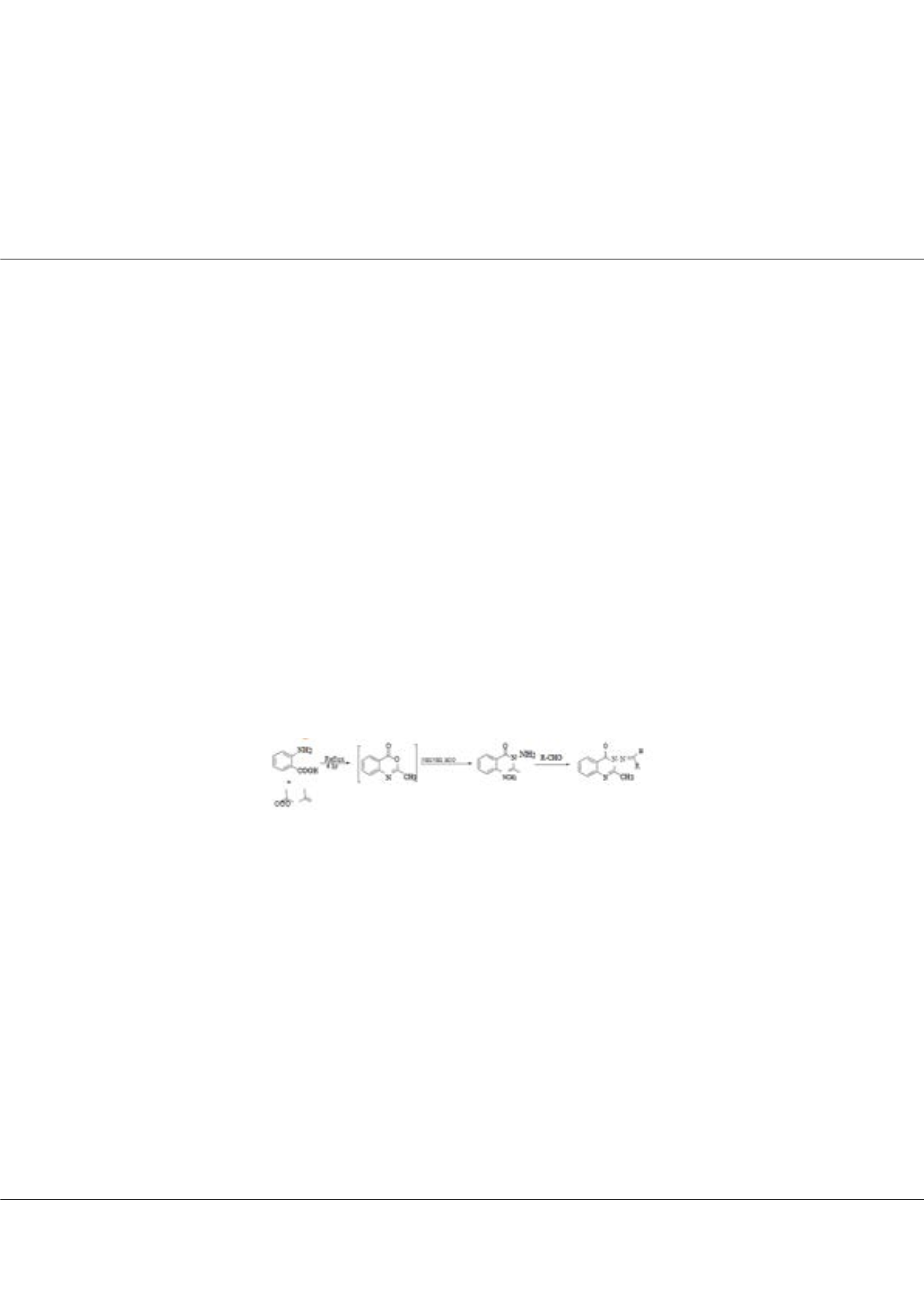
of the above facts, we prepared a new series of quinazoline-tethered Schiff bases by condensation of 3-aminoquinazolinone
with a variety of aldehydes. Predominantly, fluorinated Schiff bases were targeted owing to the property of fluorine atom to
increase in metabolic stability and bioavailability of organic scaffolds. Additionally, the molecular hybrids of quinazoline with
other pharmacophores such as quinolines, thiophene and indole were also prepared owing to their existence in biologically
significant heterocycles and drug molecules.
The full structure elucidation of all synthesized compounds was subsequently performed using 2D NMR (HMBC, HSQC,
COSY, andNOESY) techniques followed by the extensive analysis of the chemicals shifts and splitting patterns of all compounds.
The 19F being NMR active splitted both the proton and carbon nuclei of the compounds, and played a significant role in
characterization process.
Biography
Neha Manhas completed her Master’s degree in Chemistry from Durban University of Technology (South Africa), and currently pursuing her Doctorate in the School
of Chemistry and Physics at University of Kwazulu-Natal (UKZN), Durban, South Africa. Her PhD research focuses on “The synthesis, biological evaluation and
molecular modeling of novel quinazoline based heterocycles”. She has co-authored three research publications in the peer-reviewed journals.
nehamanhas09@gmail.comNeha Manhas et al., Mod Chem Appl 2017, 5:3(Suppl)
DOI: 10.4172/2329-6798-C1-006
















