
Page 26
Notes:
conferenceseries
.com
Volume 7, Issue 2 (Suppl)
J Adv Chem Eng, an open access journal
ISSN: 2090-4568
Euro Chemical Engineering 2017
November 16-17, 2017
ADVANCES IN CHEMICAL ENGINEERING AND TECHNOLOGY
November 16-17, 2017 | Paris, France
2
nd
International Conference on
Effect of initial salt concentration on acid and base production fromNH
4
Cl - NaCl solution by bipolar
membrane electrodialysis
Sinan Yapici
1
, Osman Nuri Ata
2
, M. Raşit Öner
2
, H. Emre Saygın
2
and
Neslihan Alemdar
3
1
Inonu university, Turkey
2
Ataturk university, turkey
3
Marmara university, turkey
I
n this study, a four-compartment bipolar membrane electrodialysis process was applied to produce ammonium hydroxide,
sodium hydroxide and hydrochloric acid from an aqueous salt solution consisted of ammonium chloride and sodium
chloride mixture. One of the most effective parameters in this sort of systems is the initial salt concentration. The purpose of
this work is to study the effects of the initial ammonium chloride and sodium chloride concentration on the produced amount
of hydrochloric acid, ammonium hydroxide and sodium hydroxide, the product-based salt conversion, the ion mass fluxes
transferred through the membranes. The ratio of NH
4
CI/NaCI was kept constant and the initial values of NH
4
CI/NaCI ranged
between 10/5 and 120/60 g/L. The other working conditions was maintained constant at the temperature of 25
o
C, electrolyte
flow rates of 10 L/h, at the potential difference of 10 V and the initial acid and base concentration of 0.4 M. The experiments
showed that the mass flux of the ions for the initial salt concentrations up to 40/20 (NH
4
CI/NaCI) g/L, first increased, and then
started to decrease after reaching at a maximum. Above this value, the mass flux values decreased with time, having almost
a constant value for a while and then continued to decrease again. These behaviour shows that the initial salt concentration
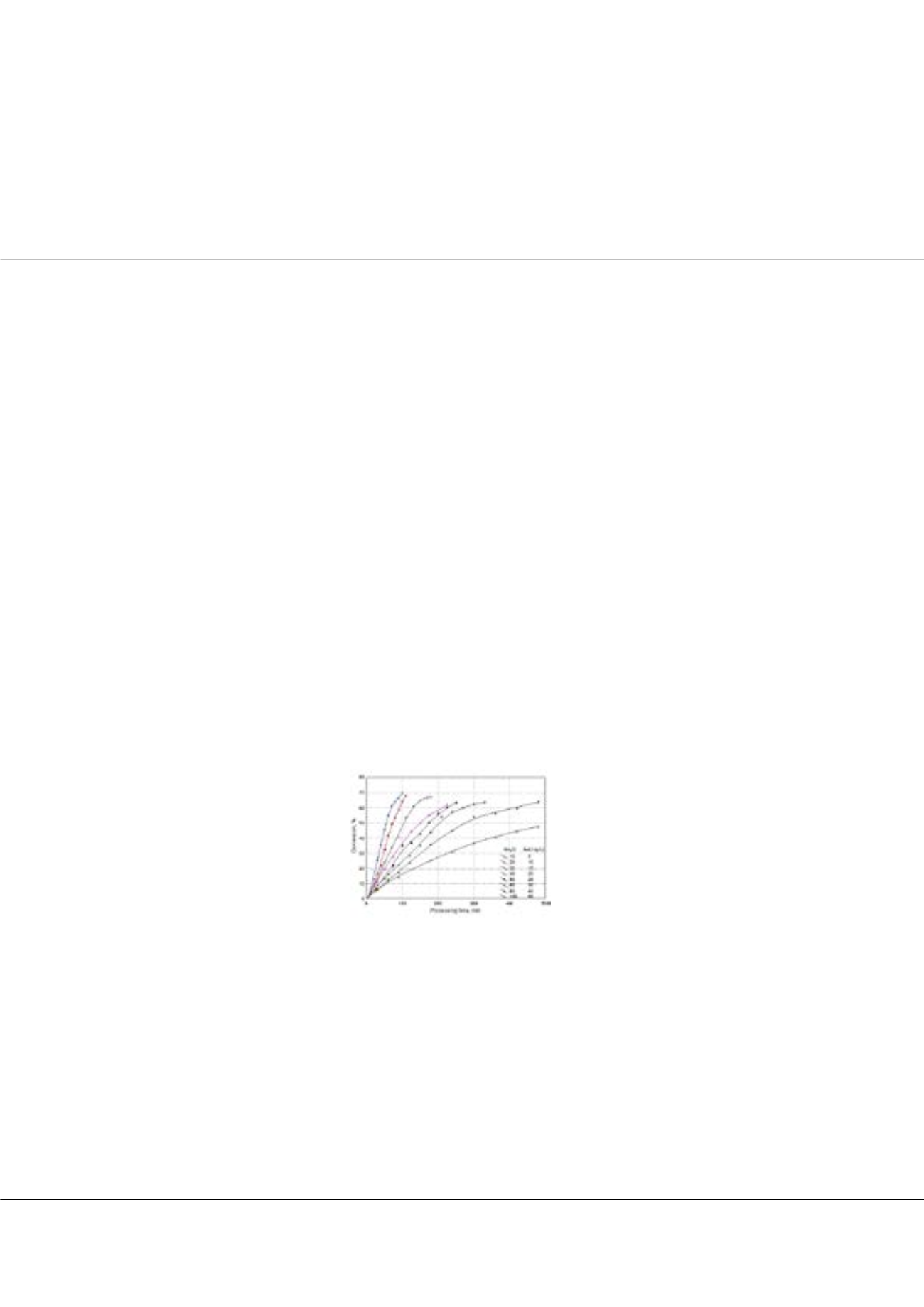
has an important effect on the mass flux of ions, that is, the rate of the process. The conversion ratios up to a value of %70 was
attained at the lowest initial salt concentration in a quite short processing time in about 100 minutes. As the salt concentration
increased, the conversion reduced down below %50 for the highest salt concentration in a longer period of 480 minutes,
showing the initial salt concentration has an important effect on conversion ratio. The consumed energy decreased with
increasing initial salt concentration, from 2.22 to1.18 kWatt∙h/kg converted salt.
Acknowledgement: Thanks to TUBITAK for its support for this work with the project no: 115Y342
Figure 1. Change of conversion with initial salt concentration.
Biography
Sinan YAPICI has his expertise in convective heat and mass transfer, electrodialysis applications and adsorption. He worked on electrochemical mass transfer
in limiting current conditions, methods of enhancing convective heat and mass transfer, and adsorption of heavy metals, and applications of electrodialysis for
synthesis of some inorganic acid and bases. He has got his PhD form Exeter university in UK, and has been lecturing in the university for more than 25 years.
sinan.yapici
@inonu.edu.trSinan Yapici et al., J Adv Chem Eng 2017, 7:2(Suppl)
DOI: 10.4172/2090-4568-C1-002
















