
Page 54
conferenceseries
.com
Volume 7, Issue 2 (Suppl)
J Adv Chem Eng, an open access journal
ISSN: 2090-4568
Euro Chemical Engineering 2017
November 16-17, 2017
ADVANCES IN CHEMICAL ENGINEERING AND TECHNOLOGY
November 16-17, 2017 | Paris, France
2
nd
International Conference on
Amino acids as gas hydrate inhibitors for offshore oil and gas industry
M Fahed Qureshi
1
, Tausif AlTamash
1
, Majeda Khraisheh
1
, Mohammad Ali Saleh
1
and
Mert Atilhan
2
1
Qatar University, Qatar
2
Texas A&M University, USA
N
atural gas hydrates are crystalline compounds that are formed under higher pressure and low temperature conditions when
small gas molecules like methane and ethane get trapped within the water molecules and form clusters. The formation
of these hydrate clusters is a major threat to offshore flow assurance, as they can lead to unwanted blockages in the subsea
pipelines and cause safety concerns. So, in order to avoid the risk of hydrate formation, the offshore oil and gas industry heavily
relies on the use of thermodynamic hydrate inhibitors (THI) like methanol and mono-ethylene glycol (MEG). These THI does
help to mitigate hydrate formation, but they are required in bulk quantity (>30 wt%), are highly flammable and cannot be
easily dispose of into the environment. So in order to avoid environmental concerns related to the use of THI, the researchers
are looking for hydrate inhibitors that are environmentally friendly, cheap and are required in low dosage. Amino acids are
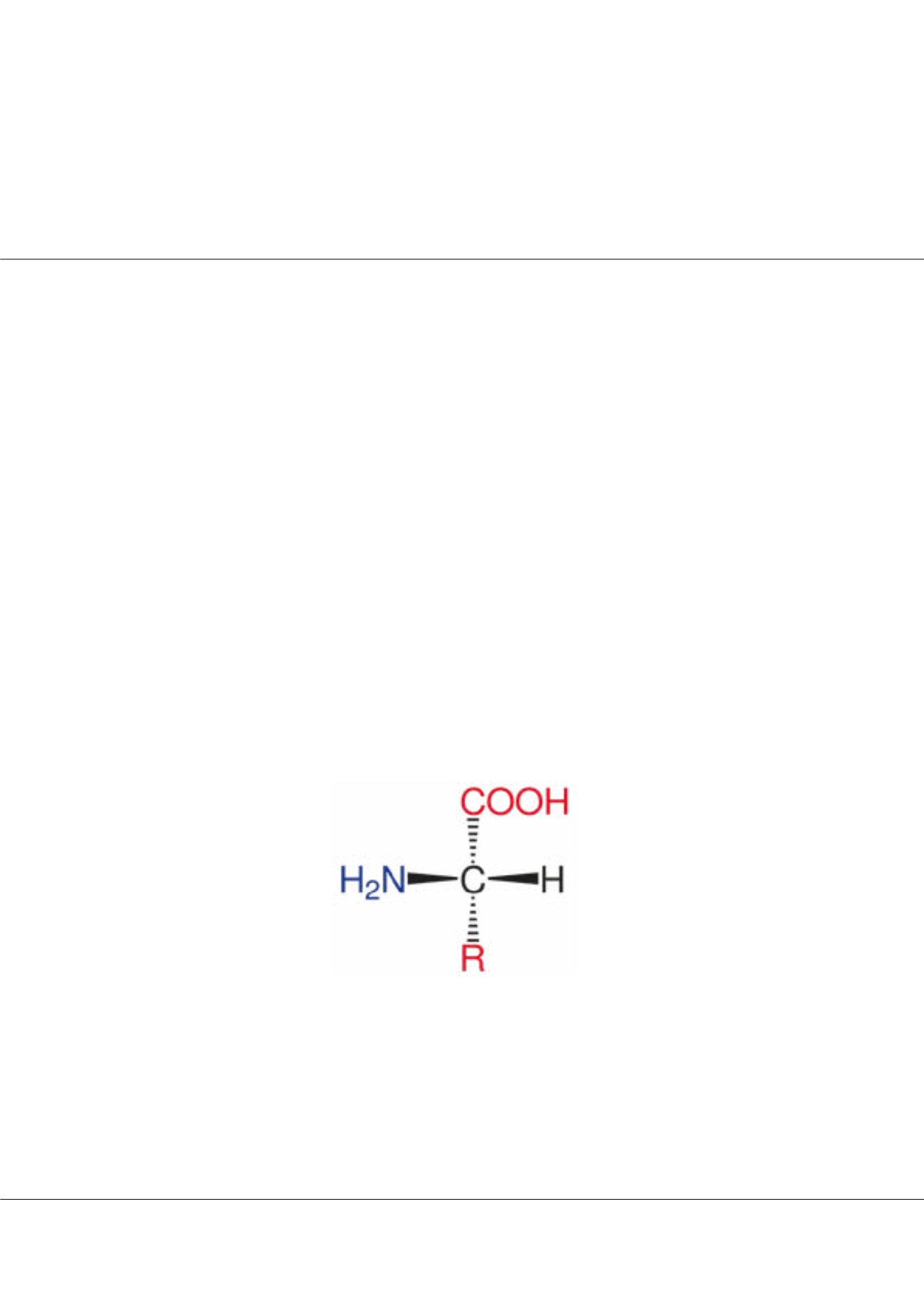
bio molecules containing amine (-NH2) and carboxyl (-COOH) functional groups, that are known to be biodegradable,
environmentally friendly, readily available and cheaper than ionic liquids. They are considered to be building blocks of life and
are widely used in the manufacturing of pharmaceutical drugs and food
products.Inthis work, the effectiveness of amino acids
as the gas hydrate inhibiter has been tested using pure methane gas and the rocking cell assembly (RC-5). The selected amino
acids (AA) included: L-Alanine, Glycine, L-Histidine, L-Phenylalanine and L-Asparagine. The experiments were carried out at
different AA concentrations (1-5wt %) and within the pressure range of 40-120 bars. The experimental results indicate that the
selected AA perform better as a kinetic hydrate inhibitor (KHI) and the addition of synergent compounds like poly-ethylene
oxide (PEO) with AA help to improve their kinetic inhibition effectiveness significantly
Acknowledgment:
Author M Fahed Qureshi would like to acknowledge GSRA. This work was made possible by GSRA # 2-1-0603-14012 from
the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility
of the authors.
Figure 1
:
Basic Amino acid structure
Biography
M Fahed
Qureshi.iscurrently a PhD fellow at Qatar University conducting research on improving effectiveness of low environmental impact gas hydrate inhibitors.
He holds MSc degree in Chemical Process Research & Development from University of Leeds UK and has previously worked with Total E& P Qatar in Acid
Stimulation project. Currently, he has three publications in the field of gas hydrates and has been awarded Graduate studies research award for his PhD work by
Qatar National Research Funds (QNRF). He enjoys writing and is interested in entrepreneurship.
Mqureshi@qu.edu.qaM Fahed Qureshi et al., J Adv Chem Eng 2017, 7:2(Suppl)
DOI: 10.4172/2090-4568-C1-002














