
Page 60
conferenceseries
.com
Volume 7
Biosensors Journal
ISSN: 2090-4967
Electrochemistry 2018
June 11-12, 2018
June 11-12, 2018 | Rome, Italy
4
th
International Conference on
Electrochemistry
Verification of photo-splitting of H
2
O to HOOH and H
2
as initial photoproducts
Shozo Yanagida
Osaka University, Japan
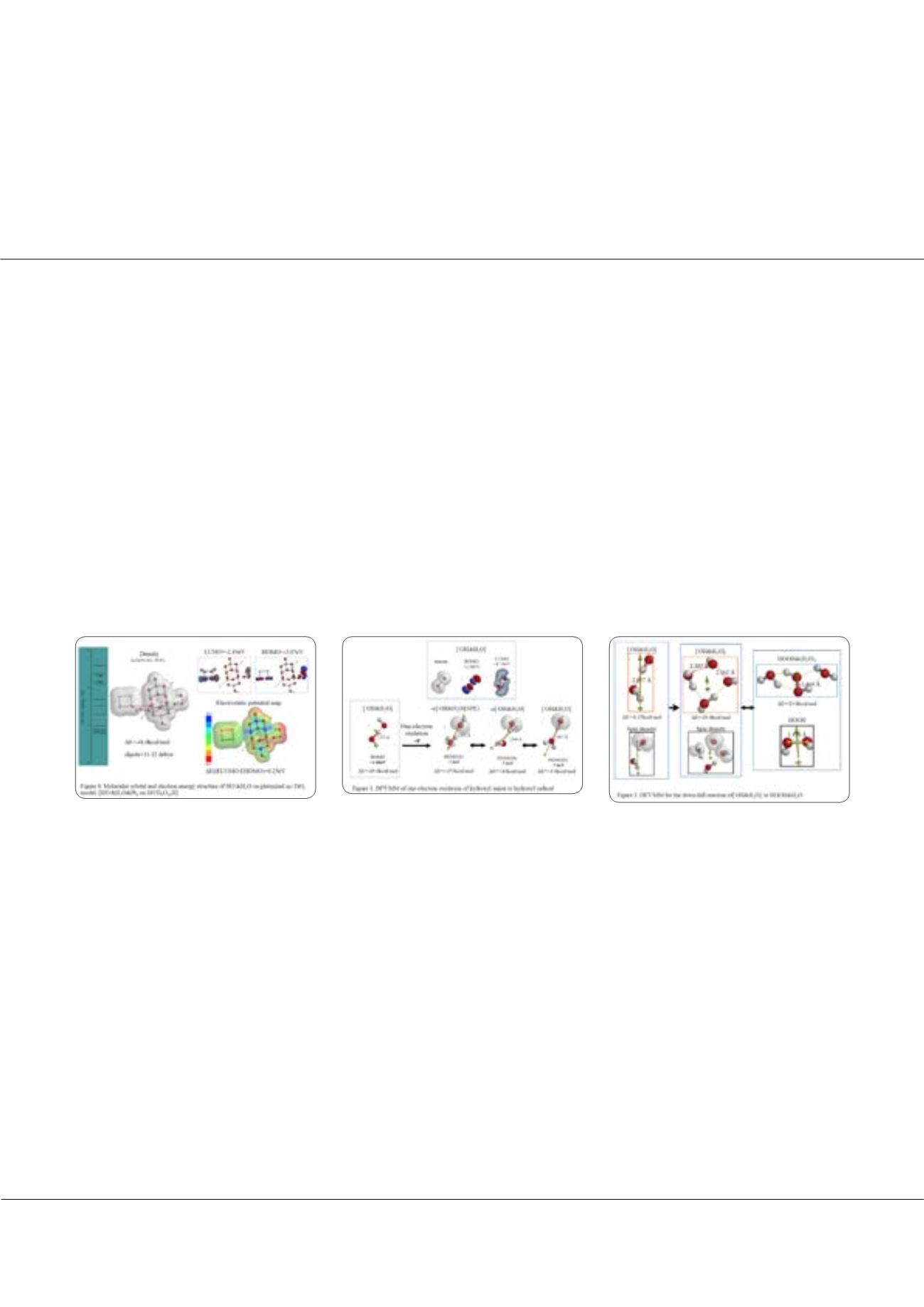
M
ost electrochemists and biochemists had a mindset that water oxidation yields oxygen molecules. However, Nosaka and
his wife reports on generation and detection of reactive oxygen species such as HO. and HOOH in photocatalysis. We
verified on the basis of density functional theory-based molecular modeling (DFT/MM) for photoelectrochemical H
2
O photo-
splitting systems that formation of HOOH only under photo-irradiated and highly negative bias conditions. Further literature
survey revealed that, in alkali aqueous solutions (pH 8~11.5), Pt-loaded nc-TiO
2
catalyzes effective H
2
O photo splitting to
HOOH and H
2
as initial products. Figure 8 shows successful DFT/MM for an aggregate induced by van-der-Waals-Coulomb
interactions (vdW&Clmb) between HOTi
9
O
18
H as a model of nc-TiO
2
photocatalyst, HO-&H
2
O as an alkali water model, and
Pt
6
as platinum cluster model. Effective photoelectron transfer is verified from [HO-&H
2
O] to Pt6 for production of H
2
on Pt
and hydroxyl radical of [HO. & H
2
O] on nc-TiO
2
. Figure 1 shows DFT/MM for exothermic one-electron oxidation of alkali
water model of hydrated hydroxide anion, [HO- & H
2
O] to hydroxyl radical of [HO. & H
2
O]. Figure 2 shows DFT/MM for
exothermic vdW&Clmb- induced dimerization of the radical of [HO. & H
2
O], verifying that oxidation of [HO- & H
2
O] to
HOOH & (H
2
O)
2
via vdW&Clmb dimerization on nc-TiO
2
. Driving force of photo splitting will be verified as due to highly
exothermic electron transfer reaction to Pt
6
on nc-TiO
2
.
Recent Publications
1. Wikipedia: “Photocatalytic water splitting”
2. Y Nosaka and A Y Nosaka (2017) Chem. Rev., 117:11302.
3. S Yanagida, S Yanagisawa, K Yamashita, R Jono and H Segawa (2015) Molecules 20:9732.
4. K Sayama and H Arakawa (1997) J. Chem. Soc., Faraday Trans. 93:1647.
Biography
Shozo Yanagida is an Emeritus Professor of Osaka University and a Research Director of Research Association for Technological Innovation of Organic Photovoltaics
(RATO) of University of Tokyo. Since he was promoted to a Professor of newly established Koza (research course) of Graduate School of Engineering in Osaka University
(1980), he had contributed to photochemical conversion of solar energy, e.g., excellent photocatalysis of both nano-sized (quantized) ZnS and poly- & oligo-paraphenylene.
When he was staying at SERI (now ENREL) as a Visiting Professor of Dr. A. Nozik’s group in 1984, he understood that organic molecules and their aggregates are kinds
of quantum dots themselves. He has his expertise in evaluation of dye-sensitized solar cells, i.e., molecular structured photovoltaics, and enthusiasm in improving photo-
conversion efficiency and long-term durability of solar cells on the basis of density-functional theory based molecular modeling.
yanagida@mls.eng.osaka-u.ac.jpShozo Yanagida, Biosens J 2018, Volume 7
DOI: 10.4172/2090-4967-C1-002
















