
Volume 10, Issue 8 (Suppl)
J Proteomics Bioinform, an open access journal
ISSN: 0974-276X
Structural Biology 2017
September 18- 20, 2017
Page 87
conference
series
.com
9
th
International Conference on
Structural Biology
September 18-20, 2017 Zurich, Switzerland
John E Baenziger, J Proteomics Bioinform 2017, 10:8(Suppl)
DOI: 10.4172/0974-276X-C1-0100
Mechanisms underlying lipid-sensing by the nicotinic acetylcholine receptor in both normal and
diseased states
T
he neuromuscular nicotinic acetylcholine receptor (nAChR) is the prototypic member of the pentameric ligand-gated
ion channel (pLGIC) superfamily, a superfamily of neurotransmitter receptors that plays a central role in information
processing in the brain. It is well documented that nAChR function is exquisitely sensitive to its lipid environment. Lipids
influence function by both conformational selection and kinetic mechanisms – they stabilize different proportions of activatable
versus non-activatable conformations, and influence the rates of transitions between the different states. In the absence of
activing cholesterol and anionic lipids, the nAChR adopts a conformation where agonist binding is uncoupled from channel
gating. Lipids likely influence the “coupling” of binding and gating
via
the lipid-exposed transmembrane
α
-helix, M4. M4
in the neuromuscular nAChR is also the site of both point and truncation mutations that alter expression and/or function
leading to congenital myasthenic syndromes. In this seminar, I will focus on the mechanisms by which the peripheral M4
transmembrane
α
-helix modulates pLGIC function. The M4 C terminus extends beyond the bilayer to interact with key
structures that link the agonist binding to the transmembrane gate – referred to here as the coupling interface. We hypothesized
that interactions between M4 and the coupling interface are essential to pLGIC function. We show here that such interactions
are essential to function in some pLGICs and do participate in lipid-sensing. In the neuromuscular nAChR, however, such
interactions between M4 and the coupling interface are less important. Instead, M4 influences function
via
a cluster of polar
residues located in the core of the transmembrane domain near the center of the lipid bilayer. Altered M4 structure leads to
changes in the energetic coupling between these polar residues, with the changes coupling ultimately propagating to both the
gating helix, M2, and the aforementioned coupling interface. Here, we map out the conformational pathway that leads from
the lipid-exposed surface of M4 to the channel gate, and thus illustrate how M4 “allosterically” modulates channel function.
Biography
John Baenziger is a professor of Biochemistry at the University of Ottawa in Ottawa, Canada. His research is focused on understanding the mechanisms by which lipids
influence nicotinic acetylcholine receptor structure and function in both normal and diseased states, with increasing focus on how lipid-nAChR interactions participate in
congenital myasthenic syndromes. Dr. Baenziger has served on the editorial board of the Journal of Biological Chemistry. He is the President of the Biophysical Society
of Canada and is Treasurer-elect of the International Union of Pure and Applied Biophysics.
john.baenziger@uottawa.caJohn E Baenziger
University of Ottawa, Canada
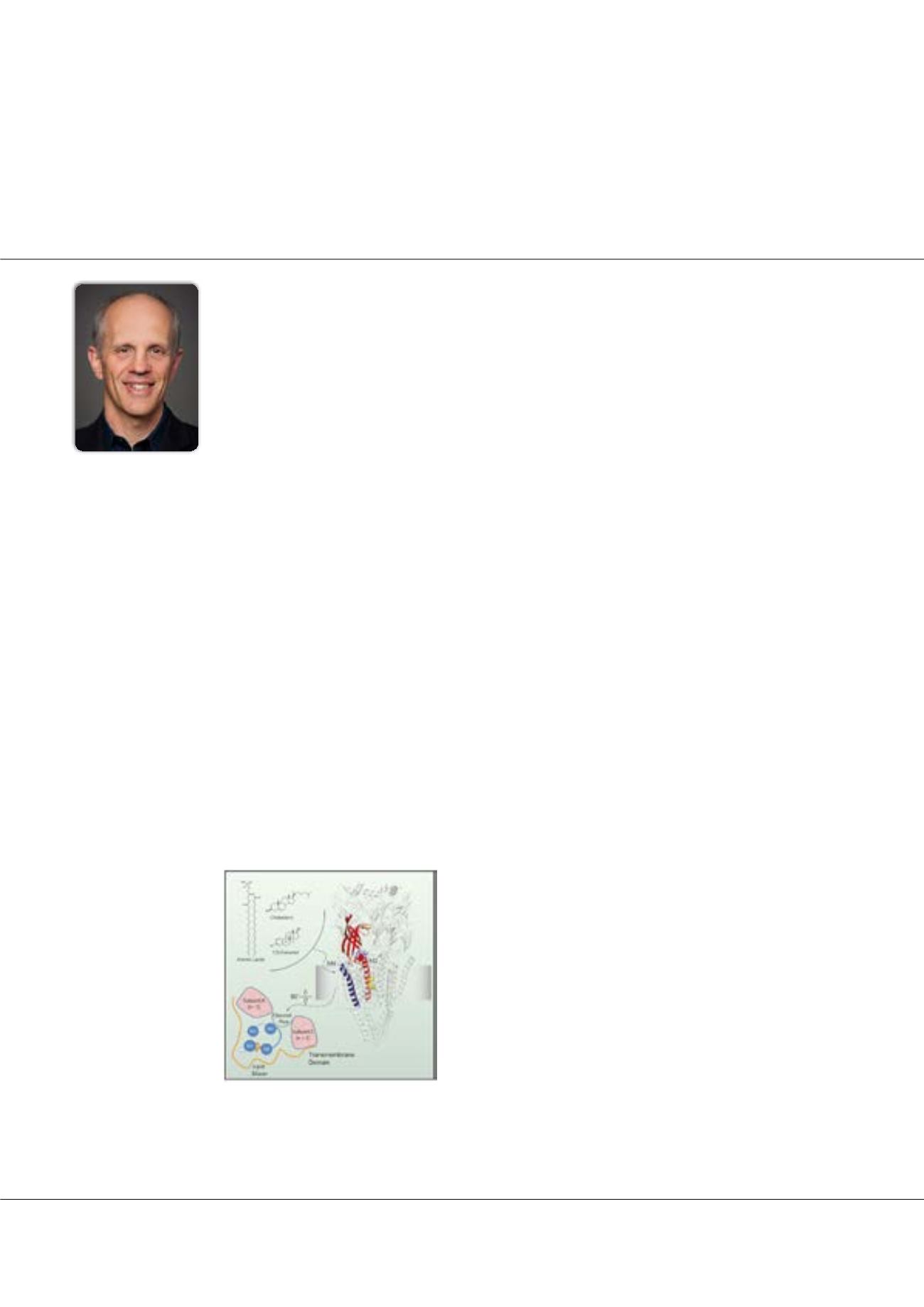
Figure1:
Some allosteric modulators, including lipids, act
via the lipid-exposed M4 α-helix of the nAChR. We eluci-
date the allosteric pathway by which this peripheral structure
influences channel gating.


















