
Page 43
conferenceseries
.com
Volume 04
Journal of Clinical Infectious Diseases & Practice
ISSN: 2476-213X
Rare Diseases Congress 2019
June 17-18, 2019
June 17-18, 2019 | Berlin, Germany
9
th
World Congress on
Rare Diseases and Orphan Drugs
Gene Silencing Approach for an orphan GNAO1-related neurodevelopmental disorder
Maryana Bardina
1,2
, Anna Polikarpova
1,2
, Elizaveta Loseva
1
, Svetlana Vassilieva
1,2
and
Tatiana Egorova
1,2
1
Marlin Biotech LLC, Russia
2
Institute of Gene Biology-RAS, Russia
G
NAO1 disorder is a fatal genetic neurodevelopmental disease characterized by epilepsy and movement
impairment that begins in early infancy. GNAO1 gene is highly expressed in the brain and certain de novo
mutations in this gene result in production of toxic protein that causes dysregulation in neuronal signaling. Currently
no effective treatment is available for this pathology. Our aim at Marlin Biotech is to find gene therapy cure for
GNAO1 disorder. Considering autosomal dominant condition of this disease, we suggest a strategy of allele-specific
gene suppression that would selectively lower levels of abnormal protein in the brain neurons and leave functional
protein unaffected. To test gene therapy approach
in vitro
, we developed an assay with expression of exogenous wild
type or mutant (c.607 G>A) GNAO1 variants in cultured cells. We screened synthetic siRNA duplexes that target
mutation site in GNAO1 RNA and downregulate expression of mutated gene through RNA interference (RNAi)
pathway. Our data demonstrates that two RNAi effectors reduce accumulation of mutant GNAO1 transcripts in
allele-specific manner. These results were confirmed at RNA and protein levels in heterozygous assay where both
wild type and mutant GNAO1 variants were introduced into cells simultaneously in 1:1 ratio to mimic heterozygous
condition of the patients. Taken together, our pilot experiments demonstrate the potential of allele-specific silencing
approach for gene therapy of GNAO1-related neurodevelopmental disorder. Our next step is designing RNAi-based
therapeutics for GNAO1 disorder that is compatible with delivery via adeno-associated virus (AAV) vectors to brain
tissues. To validate beneficial effect of AAV-RNAi technology
in vivo
, we are also developing humanized mouse
model of GNAO1 disorder using CRISPR/Cas9 technology.
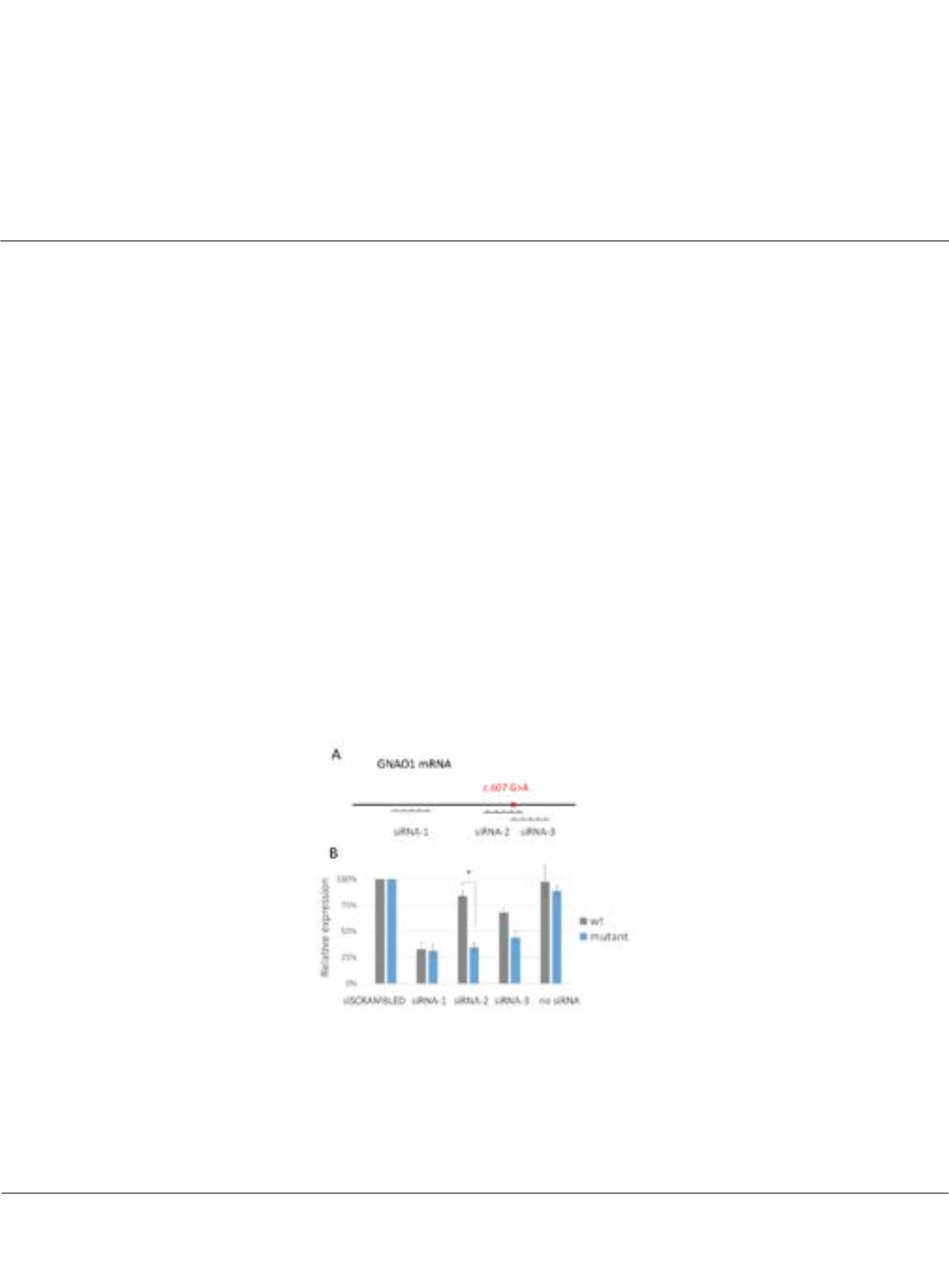
Figure 1: Allele-specific downregulation of mutant GNAO1 transcript by siRNA in cultured cells A. Position of selected siRNA target sequences in GNAO1
transcript is schematically shown. B. siRNAs targeting GNAO1 were screened in HEK293T cells expressing exogenous copies of mutant or wild type (wt)
GNAO1 siRNA-1 served as positive control and reduced expression of both GNAO1 variants to ~30%.
Biography
Maryana Bardina has obtained her PhD in Molecular Virology at Lomonosov MSU, Moscow, Russia and completed training in viral vector design and gene
suppression technologies at ICGEB, Trieste, Italy. She has joined Marlin Biotech in 2016 and developed methods for AAV production and purification. From
November 2017, she leads the project on GNAO1 disorder aiming at finding gene therapy cure for this neurological disease.
m.bardina.marlin@gmail.comMaryana Bardina et al., J Clin Infect Dis Pract 2019, Volume 04
















