
Volume 6
Journal of Infectious Diseases & Therapy
ISSN: 2332-0877
Infection Prevention 2018
December 06-07, 2018
Page 41
conference
series
.com
December 06-07, 2018 | Valencia, Spain
14
th
World Congress on
Infection Prevention and Control
Ionel Victor Pătraşcu, J Infect Dis Ther 2018, Volume 6
DOI: 10.4172/2332-0877-C6-052
Biological products pi-2a II-oral and topical treatment of pediatric psoriasis
Introduction:
Psoriasis is a chronic, immune-mediated, inflammatory skin disease, affecting 1–3% of the white population.
Although two incidence peaks have been suggested (in adolescence and adulthood), the onset may occur at any age, including
childhood (1). Guidelines for pediatric psoriasis treatment are lacking due to side effects of therapies approved for treatment in
adult patients. In this study we show that the treatment of psoriasis-affected children with Avian Immunologically Active Proteins
(PI-2A) obtained using our patented technology under Romanian brands IMUNOINSTANT and IMUNOVIP (2-4) was followed
by impressive resolution of clinical symptoms without reported side effects. PI-2A are a novel class of biological agents that target
specific mediators of inflammation as well as antimicrobial resistant (AMR) microorganisms. Multiple studies have confirmed their
efficacy in the treatment of psoriasis in adults (3). “Standard” PI-2A contain antibodies against a panel of 22 microbial antigens; they
are formulated as sterile solution, sterile spray, granules, ointments, healing oily liquid, healing granules, healing tablets with easily
adsorbable collagen VII, sterile yolk suspension. “Personalized” PI-2A are prepared similarly from pathological materials taken from
psoriasis plaques of individual patients
Study design:
15 children aged 3 to 12 years presenting severe psoriasis vulgaris were treated with “standard” PI-2A formulated as
oral preparations (sterile solution, granules) and topic preparations (healing oily liquid, sterile yolk suspension) during a 3 months
session. Subjects were excluded if presenting history of allergic reaction to egg-derived products. Evaluated parameters were: severity
of skin lesions, presence and evolution of fingernail pitting.
Results:
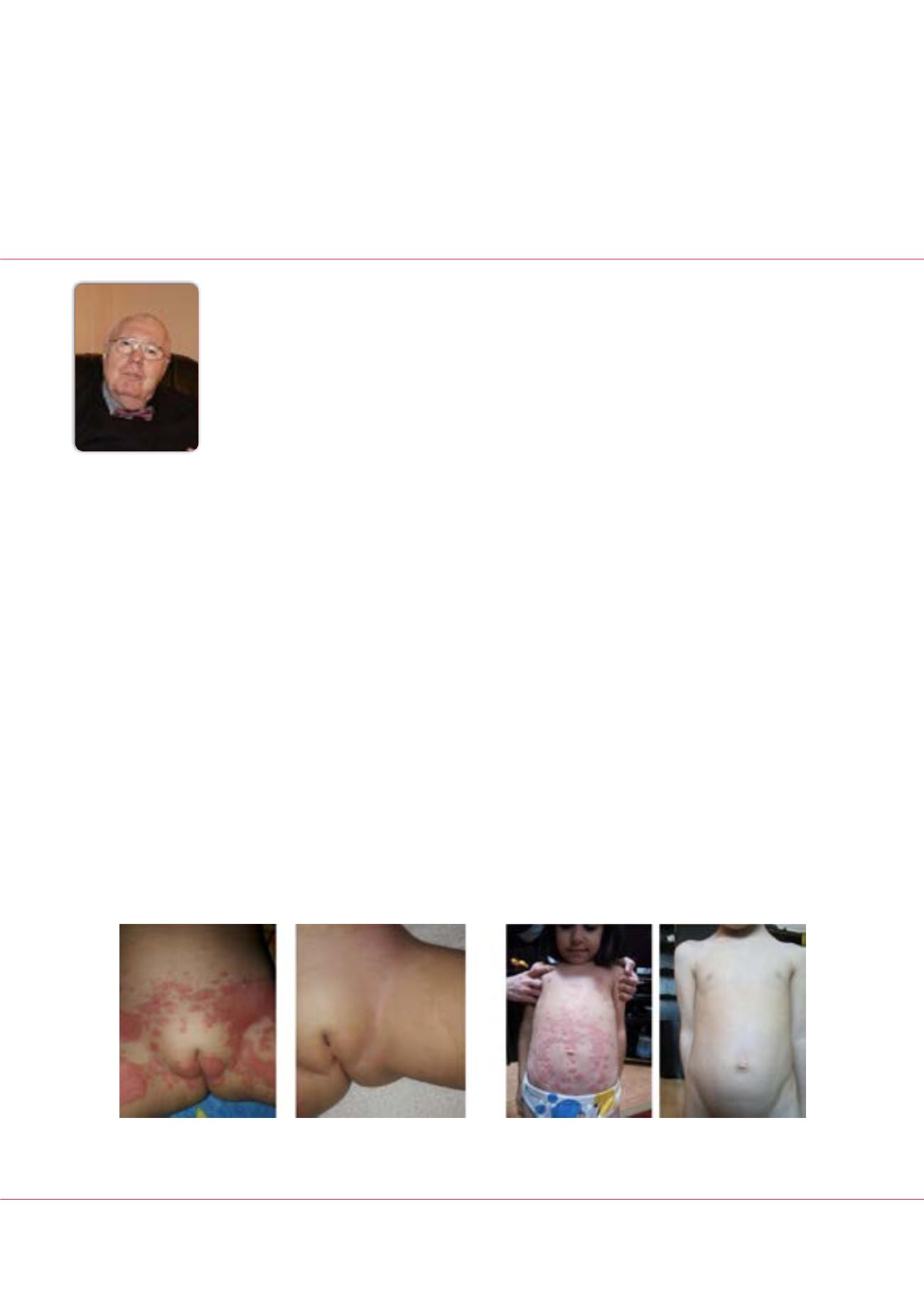
The evolution of skin and fingernail lesions was favourable for the entire group of studied subjects (Figure 1 shows two
representative cases). There were no reports of intolerance or adverse reactions to the oral and topic use of PI-2A.
Conclusion:
PI-2A are an important too in the treatment of pediatric psoriasis.
Ionel Victor Pătraşcu
Activeimmunity srl., Romania


















