
Page 53
conferenceseries
.com
Volume 7
Biosensors Journal
ISSN: 2090-4967
Electrochemistry 2018
June 11-12, 2018
June 11-12, 2018 | Rome, Italy
4
th
International Conference on
Electrochemistry
Oxygen evolution reaction at the surface of nickel cobaltites: The impact of surface restructuring
phenomena on the activity
Aurélien Habrioux, Ismail Abidat, Clément Comminges, Claudia Morais, Teko Napporn
and
Boniface Kokoh
University of Poitiers, France
T
he storage of intermittent renewable energies requires the implementation of efficient energy storage systems. These systems
must allow converting renewable energies into sustainable energetic vectors (hydrogen, electron). For this purpose, the oxygen
evolution reaction (OER) plays an important role. OER possesses a sluggish kinetics that can be enhanced by using a catalyst
exhibiting reliable surface composition and morphostructural properties. To limit the use of scarce noble metals, the synthesis of
effective 3D transition metal oxide-based catalysts is of interest. As activity and stability of materials depend on their composition
and morphostructural properties, the synthesis of well-defined catalysts is of utmost importance. To this end nanocasting
approach constitutes an interesting pathway. In this study, NixCo
3
-xO
4
materials have been synthesized by replicating ordered
mesoporous silica templates. Materials were investigated using numerous physico-chemical techniques such as x-ray induced
photoelectron spectroscopy (XPS), high resolution transmission electron microscopy, x-ray diffraction and Raman spectroscopy.
Evidences from XPS and Raman measurements reveal that the different catalysts surfaces are hydroxylated. A particular attention
was paid to restructuring phenomena occurring upon potential cycling and responsible for greatly improving the OER activity.
These restructuring phenomena were evidenced using post-mortem Raman spectroscopy and XPS. It was observed that the
intrinsic activity of the different restructured catalysts depends on the incorporated nickel amount and correlates with the CoIII/
CoIV peak potential. The modulation of CoIII/CoIV peak potential is explained by changes in the chemical environment of
surface Co atoms and results in the formation of nickel/cobalt oxy-hydroxide. Nickel indeed modulates the electronic properties
of the Co active site and allows improving the OER activity of electrode materials. The catalysts described in this presentation are
moreover very efficient since after surface restructuring, the overpotential at 10
mA.cm-2
is as low as 310 mV.
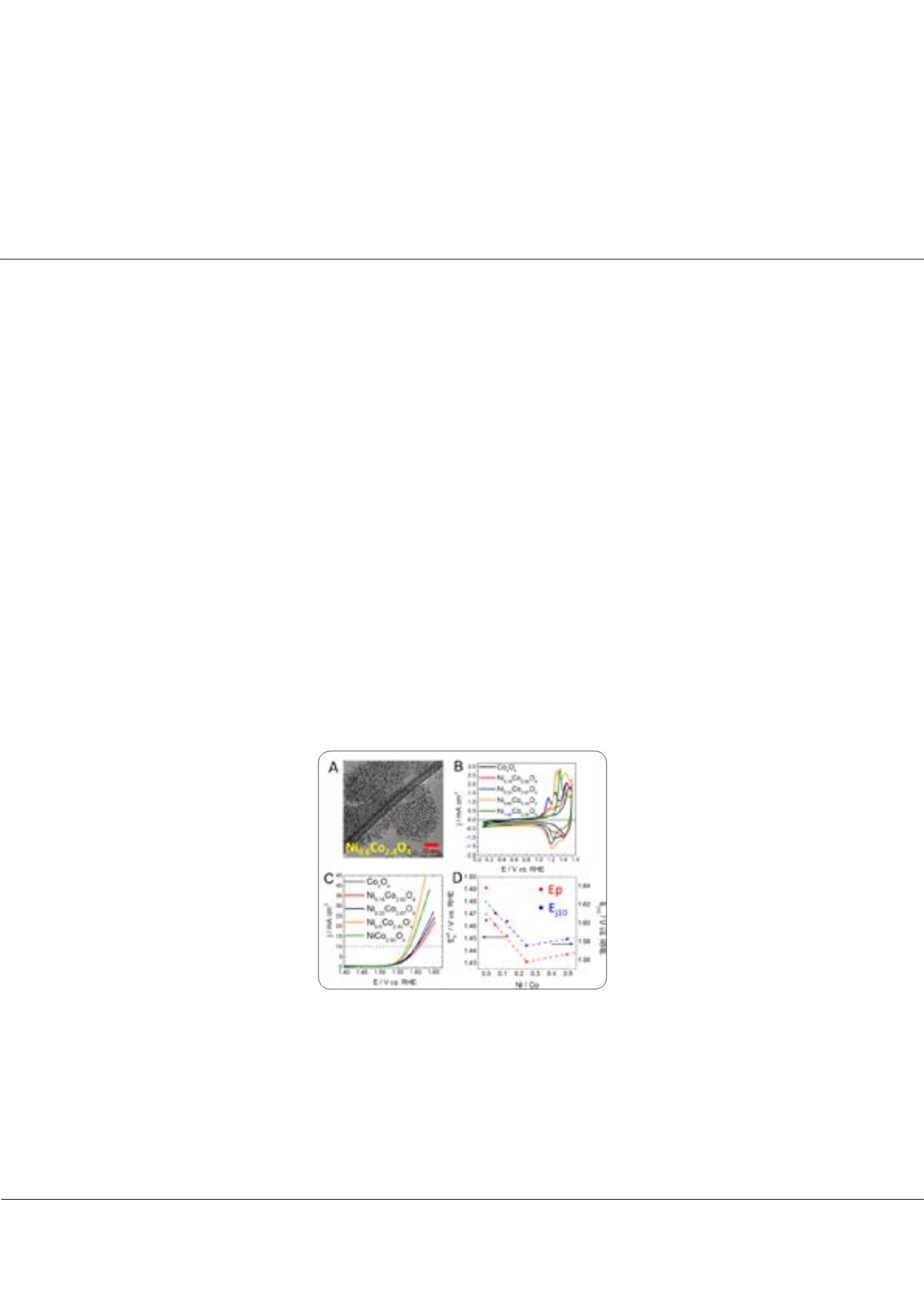
Figure 1: A)
TEM image of Ni
0.6
Co
2.4
O
4
catalyst.
B)
Cyclic voltammograms of Ni
x
Co
y
O
4
recorded in a 1 mol L
-1
KOH electrolyte. Scan rate 20 mV s
-1
C)
OER polarization curves recorded with Ni
x
Co
y
O
4
in a 1 mol L
-1
KOH electrolyte. Scan rate 5 mV s
-1
D)
Correlation between position of CO
3
+/CO
4
+ redox transition (red dots) and OER overpotential at 10 mA cm
-2
(blue dots)
Aurélien Habrioux et al., Biosens J 2018, Volume 7
DOI: 10.4172/2090-4967-C1-002
















