
Page 52
conferenceseries
.com
Volume 7
Biosensors Journal
ISSN: 2090-4967
Electrochemistry 2018
June 11-12, 2018
June 11-12, 2018 | Rome, Italy
4
th
International Conference on
Electrochemistry
(Electro)chemical water disinfection – challenges for the 21
st
century
M E Henry Bergmann
Anhalt University of Applied Sciences, Germany
W
hereas worldwide hundreds of millions of people do not have access to safe drinking water, in developed countries
current research and practice are oriented towards problems of micropolutants and disinfection by-products formed
at µmol-per liter level. Another special subject of activities is the low-chemical disinfection of process waters in recirculation
and cooling systems. Although direct electrochemical drinking water disinfection is applied for decades of years, conditions
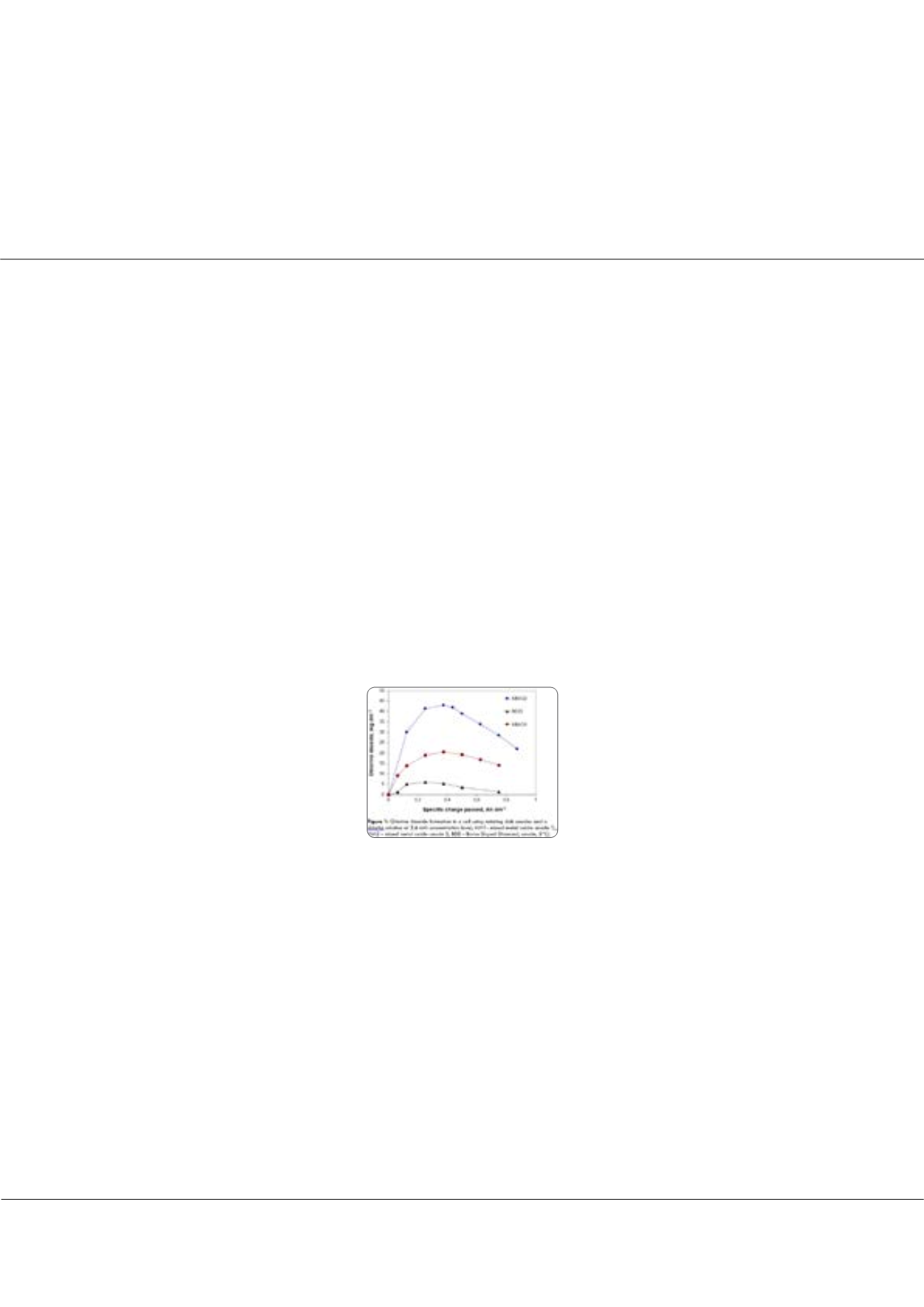
for avoiding overchlorination and disinfection by-products could only be clarified in the recent years. A new approach is the
electrochemical generation of chlorine dioxide (ClO
2
) at mM concentration level. It is well known that ClO
2
has much lower
organic by-product formation potential compared to free active chlorine. The challenge is to minimize chlorite, chlorate and
perchlorate in the solutions obtained. The high chlorite reactivity often causes maxima in ClO
2
formation. Highly active anodes
dramatically reduce the generation of chlorine dioxide due to parasitic reactions. Solutions for inorganic electrolysis and
disinfection by-products can be found by analyzing and studying influence parameters such as temperature, electrode material,
counter electrode material and cell construction. ClO
2
--to-ClO
2
yields of 75% are possible at the moment. Furthermore, it is
discussed if combined methods may contribute to lower by-product formation. A simple variant is the combined chlorine-
chlorine dioxide formation. The combination of ozone and chlorine dioxide
in situ
is another interesting option. First own
experiments have shown that all initial chlorite can be converted to chlorine dioxide. Lower temperatures in the range of 5 are
preferred reaction conditions. It can be stated that in the future improved regulations and inline analysis methods have to be
applied for safer disinfection. Means of digitalization could support the process.
Recent Publications
1. Kähkönen E andNordströmK (2008) Toward a nontoxic poison: current trends in (EuropeanUnion) biocides regulation.
Integr Environ Assess Manag 4:471-477.
2. Final Research Report (2012) Save Drinking Water By Using Inline Electrolysis. Supported by DVGW (10/02/08 and
DBU (AZ 25386), Bad Elster, Berlin, Dresden and Köthen.
3. Bergmann M E H and Koparal A S (2005) Problems of chlorine dioxide formation during electrochemical disinfection.
Electrochim Acta 50:5218-5228.
4. Haas C N (1990) Disinfection. Pontius F W (Ed.), Water Quality and Treatment, McGraw-Hill Inc: 877-932.
5. Lindner K, Lew J, Carter B and Brauer R (2003) Avoiding chlorite: chlorine and ClO
2
together to form fewer DBDs.
Opflow 32:24-26.
Biography
M E Henry Bergmann has his 35-years expertise in electrochemical engineering that is recently focused on methods of drinking and process water disinfection. His
technological approach for small and medium-size treatment devices respects the new European Biocide Regulation and the practical needs as well - against the
background of avoiding or minimizing chlorination, and the formation of hazardous by-products. New (electro)chemical technologies are suggested basing on the use of
chlorine dioxide, physically or electrochemically generated ozone, and combined methods.
henry.bergmann@hs-anhalt.deM E Henry Bergmann, Biosens J 2018, Volume 7
DOI: 10.4172/2090-4967-C1-002
















