
Volume 8
Journal of Biotechnology & Biomaterials
ISSN: 2155-952X
Biotech Congress 2018 & Enzymology 2018
March 05-07, 2018
Page 38
conference
series
.com
JOINT EVENT
20
th
Global Congress on
Biotechnology
3
rd
International Conference on
Enzymology and Molecular Biology
&
March 05-07, 2018 London, UK
Magali Remaud Simeon, J Biotechnol Biomater 2018, Volume 8
DOI: 10.4172/2155-952X-C2-090
Mixing enzyme discovery with engineering for sucrose-derived bioproducts: The case of GH13 and
GH70 polymerases
T
he exploration of the natural diversity, through data mining, functional genomics and/or metagenomics is an efficient mean
to discover enzymes showing new functions or improved performances. These approaches can be further completed or run
in parallel with semi-rational protein engineering based on structure/function studies or directed molecular evolution inspired
from nature. Which of these alternatives are the best ones, in terms of effort, rapidity and efficiency? This is an open question
to which a definite answer can be hardly formulated a priori. For illustration, we will take a few examples from our most recent
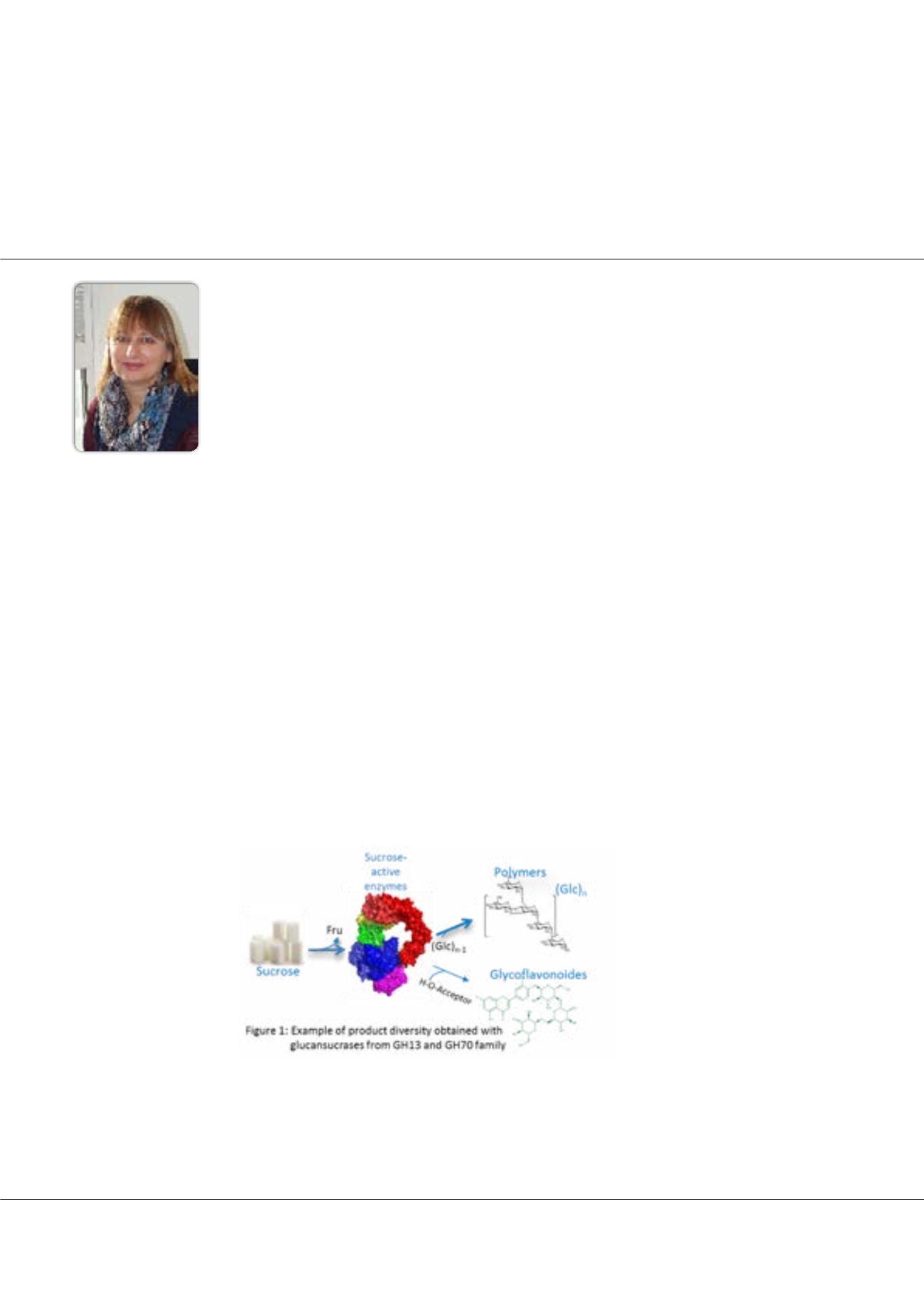
work on glucansucrases from GH13 and GH70 families. These enzymes are naturally very efficient transglucosylases. They
use sucrose as substrate and catalyze polymerization of its glucosyl units as a main reaction. Depending on their specificity,
structures varying in size as well as in glycosidic linkage types can be obtained, thus giving access to an interesting panel of
biopolymers. A campaign of genome sequencing and data mining allowed the isolation of atypical enzymes with new product
specificities. In particular, a hyper efficient polymerase producing a gel-like polymer and, in contrast an enzyme synthesizing
directly from sucrose a polymer of well-controlled lowmolar mass could be characterized. Structure-function studies combined
with mutagenesis assays allowed us to decipher some of the molecular mechanisms behind the control of the polymer size and
enzyme processivity. Another key property of these catalysts is coming from their ability to glucosylate a broad spectrum of
hydroxylated molecules. Computational protein design, structurally-guided engineering and also random approaches such as
neutral evolution was implemented for a fine tuning of their acceptor specificity toward non-natural acceptors such chemically
protected disaccharides for vaccinal applications, polyol, flavonoids, or various chemicals. These various approaches will be
described and discussed with regard to the engineering objectives.
Recent Publications
1. Claverie M et al. (2017) Investigations on the determinants responsible for low molar mass dextran formation by
DSR-M dextransucrase. ACS Catal. 7(10):7106-7119.
Magali Remaud Simeon
INSA - Université de Toulouse, France
















