
Volume 9
Journal of Biotechnology & Biomaterials
ISSN: 2155-952X
Biomaterials 2019
February 25-26, 2019
Page 26
conference
series
.com
February 25-26, 2019 | London, UK
4
th
Annual Conference and Expo on
Biomaterials
Gianfranco Peluso, J Biotechnol Biomater 2019, Volume 9
DOI: 10.4172/2155-952X-C1-111
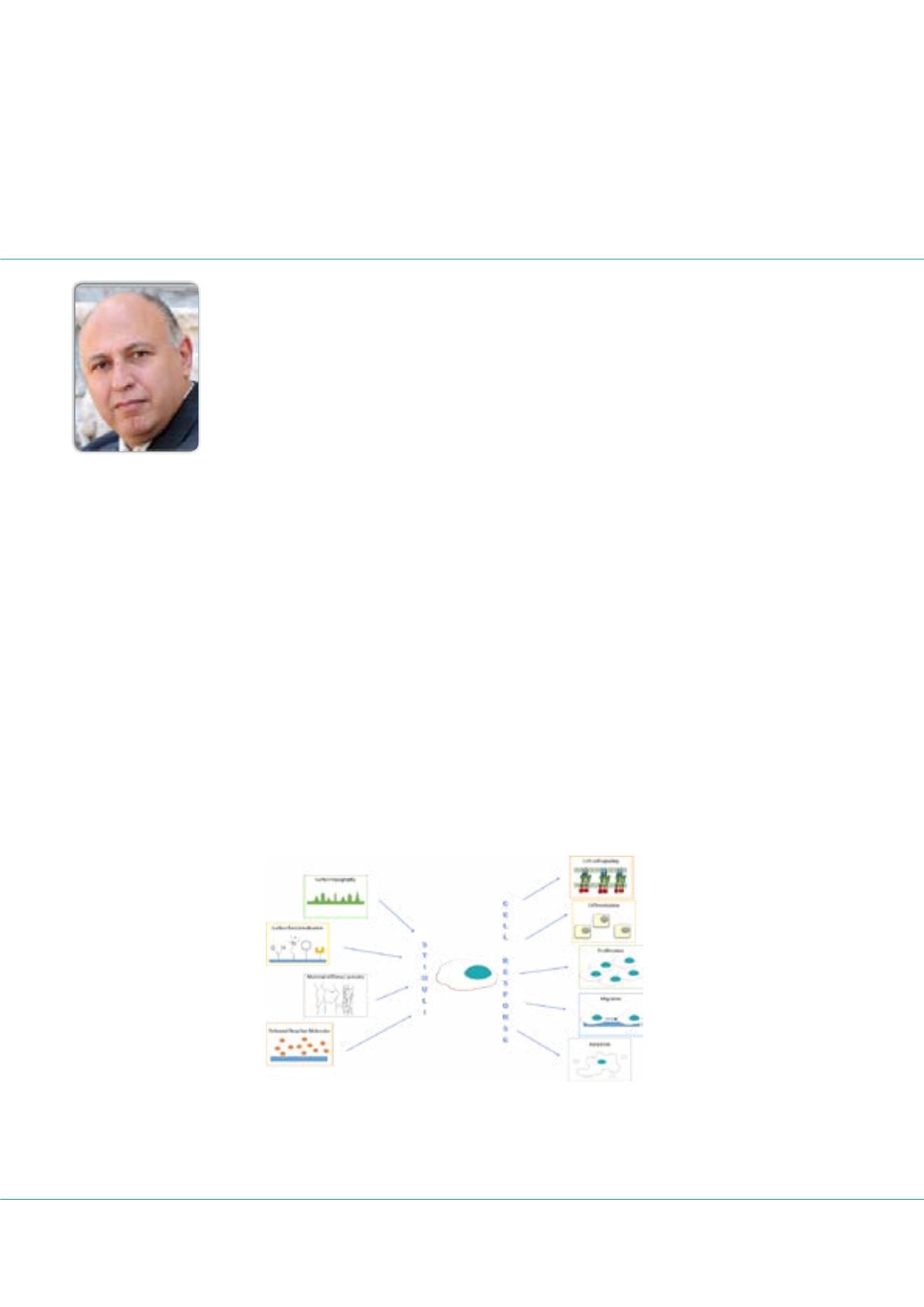
Cell/biomaterials interaction
T
he cell/material interaction is a complex, dynamic process in which the cell and the material synergistically influence the fate
of the cell. Indeed, both materials intrinsic (i.e. topography, charge, ζ-potential, and contact angle) and extrinsic properties (i.e.
surface functionalization, crystallinity, etc.) played a pivotal role in dictating the type and strength of the biological responses (Figure
1). Furthermore, the ability of biomaterials to release bioactive molecules (i.e. resveratrol, fluoride, etc.) expands the possibilities to
control cell-cell interactions and/or intracellular signal transduction. Our recent research demonstrated a functional role of charged
polymers in altering or supporting the osteogenic differentiation of mesenchymal stem cells (MSCs) through the modulation of
the ephrinB2/EphB4 interaction. Indeed, cell-cell signaling pathways that lead to efficient differentiation of stem cells include the
interaction of Ephrin ligands (ephrinB2) with Eph receptors (EphB4). For the first time we have shown that high charged polymers
can affect the Eph/ephrin interaction between neighboring cells inhibiting the MSCs osteogenic differentiation via the perturbation
of the bidirectional signaling. In contrast, low charged polymers modulate the differentiation of MSCs into an osteocyte lineage via
cell-cell ephrinB2/EphB4 signaling.
Moreover, we demonstrated that electrospun PCL and PLA nanofibers loaded with resveratrol (RSV) differently modulate DPSCs
osteoblast differentiation and inhibit osteoclastogenesis depending on their RSV release kinetics. Our results indicate that the
slow and continuous RSV release from PLA was able to modulate both osteoblast and osteoclast differentiation representing a
promising material for the preservation of post-extraction integrity of alveolar socket. Taking together, our results highlight that
rationally designed materials can give rise to biomaterials able to modulate functional aspects of biological signaling. Furthermore,
understanding the mechanisms by which cells respond to external stimuli could be a successful strategy i.e. in cancer therapy,
regenerative medicine, etc.
Figure 1: Schematic illustration of cell/biomaterials interaction. Intrinsic and extrinsic materials properties could affect cell fate
and tissue development inducing a cell response.
Gianfranco Peluso
IRET-CNR, Italy
















