
Page 56
conferenceseries
.com
Volume 7
Innovative Energy & Research
ISSN: 2576-1463
Advanced Energy Materials 2018
August 13-14, 2018
August 13-14, 2018 | Dublin, Ireland
20
th
International Conference on
Advanced Energy Materials and Research
Materials for thermochemical energy storage: Experimental investigation of cycling stability
M. Gollsch, J. Stengler, M. Spindler
and
M. Linder
Institute of Engineering Thermodynamics,German Aerospace Center (DLR), Germany
T
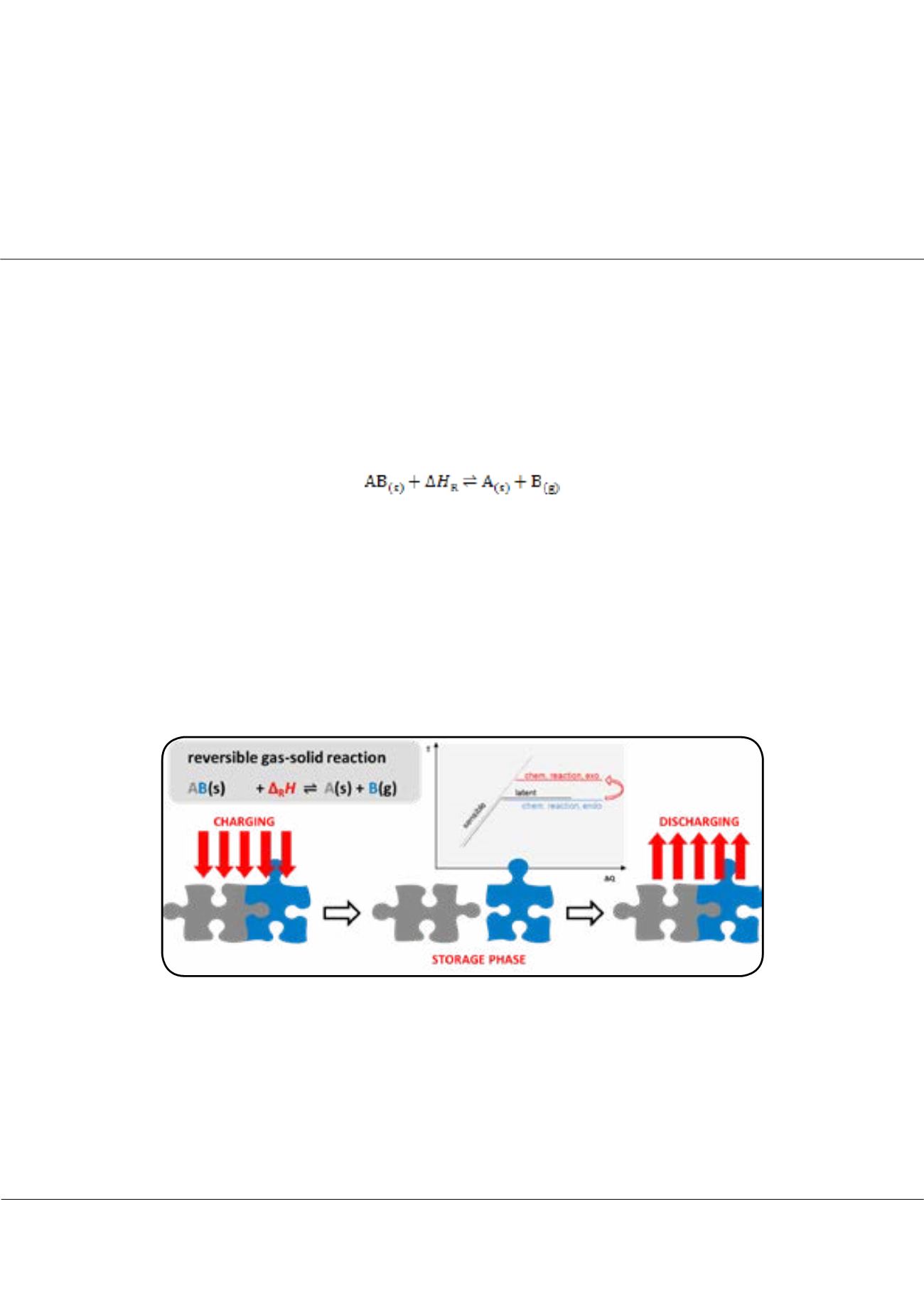
hermochemical energy storage (TCS) uses the reaction enthalpy of reversible chemical reactions. This storage technology
contains a so far largely untouched potential: in comparison to sensible and latent thermal energy storage, TCS offers
potentially higher storage densities, the possibility of long-term storage as well as the option to upgrade the thermal energy.
This upgrade can be realised if the reaction system consists of a solid and a gaseous component. For these gas-solid reactions
with the generic equation
the equilibrium temperature is dependent on the reaction gas partial pressure: the higher the partial pressure, the higher the
reaction temperature. Consequently, the charging of the storage can take place at lower temperatures than the discharging
by adjustment of the reaction gas partial pressure. Currently, a number of water vapour-solid reactions are investigated as
thermochemical storage materials [1-4]. Apart from a general suitability of a reaction system for thermochemical storage,
special attention has to be paid to the cycling stability of the reaction. This is often done using thermogravimetric analysis [5].
However, past scale-ups have shown that behaviour of bulks differs from that of analysis amounts [6]. The bulk’s changing
properties, however, have proven to be crucial for storage reactor design. The investigation of the cycling stability and reaction
behaviour of reacting solid bulks has been our motivation to design and build a cycling test bench. In this experimental
setup the gaseous reaction partner is water vapour and can be provided at pressures between 5 kPa and 0.5 MPa. Reactor
temperatures can be up to 500 °C. The aim of the presented studies is the automated cycling of about 100 ml solid storage
material of reaction systems that have previously shown promise at analysis scale.
Recent Publications
1. Gutierrez, A., Ushak, S., Linder, M. (2018) High Carnallite-Bearing Material for Thermochemical Energy Storage:
Thermophysical Characterization. ACS Sustainable Chemistry and Engineering 6(5):6135-6145
2. Afflerbach, S., Kowald, T., Trettin, R. (2017) Phase transformations during de- and rehydration of scholzite
CaZn
2
(PO
4
)
2
•2H
2
O. Journal of Solid State Chemistry 254:184-194
3. Stengler, J., Ascher, T., Linder, M. (2017) High temperature thermochemical heat transformation based on SrBr
2
. 12th
IEA Heat Pump Conference, 15th-18th May, 2017, Rotterdam
M. Gollsch et al., Innov Ener Res 2018, Volume 7
DOI: 10.4172/2576-1463-C1-002
















