
Page 51
conferenceseries
.com
Volume 8
Journal of Biotechnology & Biomaterials
ISSN: 2155-952X
Pharma Biotech 2018
December 10-11, 2018
December 10-11, 2018 | Rome, Italy
23
rd
International Conference on
Pharmaceutical Biotechnology
Development of monodisperse magnetic porous/hollow nanostructures for biomedical applications
Kyo-Seon Kim, Sang-Hyeok Yoon
and
Nguyen D T
Kangwon National University, South Korea
R
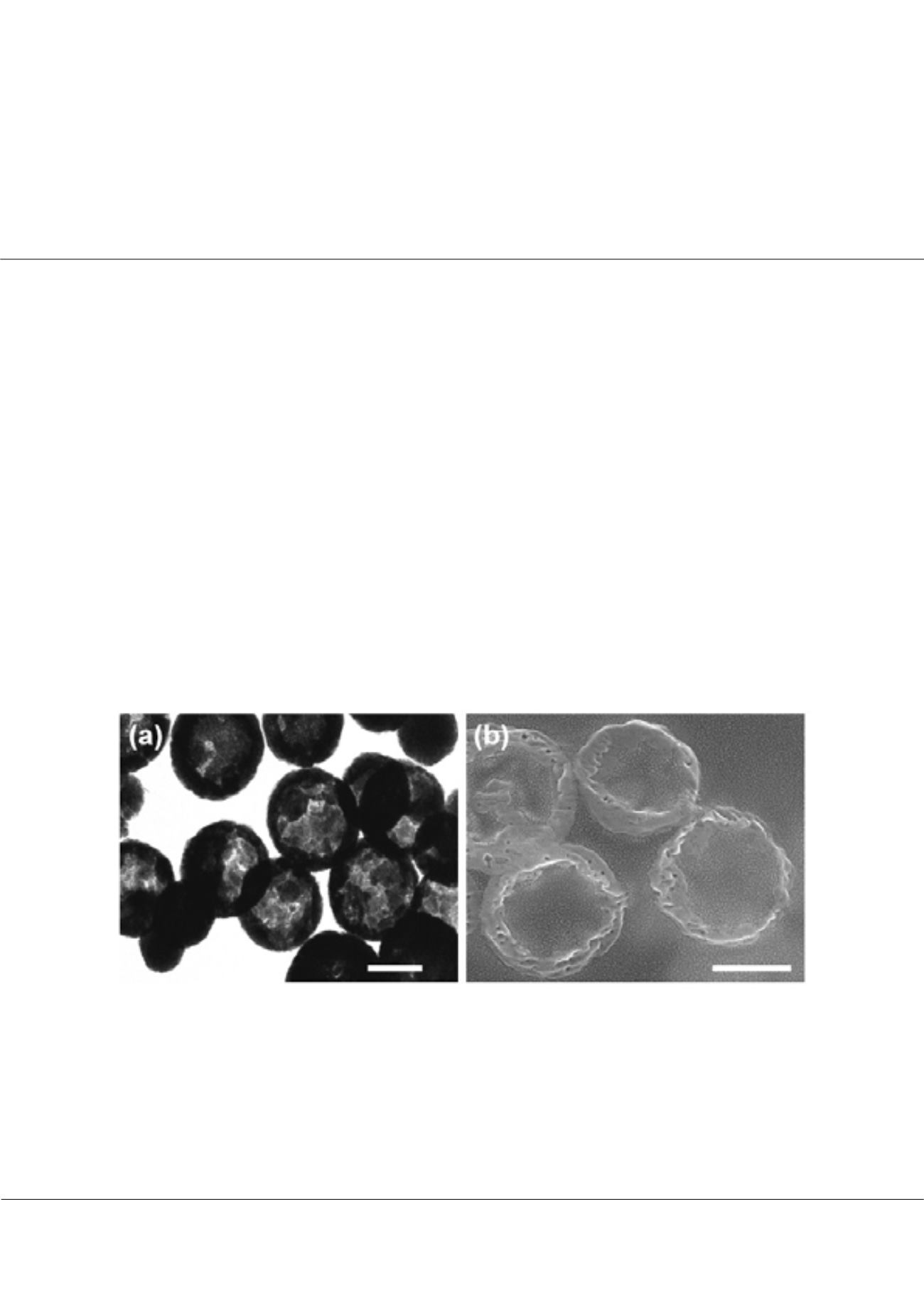
ecently, studies on therapeutic applications of the magnetic nanoparticles have gained more momentum. The porous/
hollow structure also exhibited a great potential to encapsulate small drug molecules. Once inside the porous structures,
small drug molecules would be shielded by the shell from fast reaction/deterioration in biological solutions. In our research,
monodisperse magnetite Fe
3
O
4
porous/hollow nanoparticles were successfully synthesized through one-pot solvothermal
process without any surfactant and template as shown in Figure The Fe
3
O
4
porous/hollow nanoparticles consisted of numerous
tiny grains. Those particles were ferromagnetic with high saturation magnetization. The Fe
3
O
4
porous/hollow nanoparticles
were synthesized controllably with tunable particle size and porosity by adjusting the initial concentrations of Fe precursor
and ammonium acetate. The formation mechanism of the magnetite hollow spheres comprised simultaneous chemical and
physical processes including the formation of numerous tiny grains, the spherical assembly of those grains and the chemical
conversion coupled with the relocation of the grains. The chemical conversion including a partially reductive reaction of the
Fe (III) compounds and subsequent hydrolysis and dehydrolysis reactions of the Fe (III) and Fe (II) compounds to generate
Fe
3
O
4
caused the non-uniformities of tiny grains and the empty spaces within the spherical assemblies and thus enhanced
the outward migration and relocation of the core grains toward the outer layer, resulting in the formation and expansion
of the hollow core structure. The porous/hollow nanoparticles could be further coupled with a specific targeting agent and
be concentrated around the area of interest, where drug molecules would be released either chemically via a pH control or
physically through a magnetic stimulation and activation. Such a controlled drug release warranted the multifunctional porous/
hollow nanoparticles a new class of carriers for simultaneous diagnostic and therapeutic applications.
Figure: Representative (a) TEM image and (b) SEM image for the cross-section of the Fe
3
O
4
porous/hollow nanospheres.
Recent Publications
1. Nguyen D T and Kim K S (2013) Template-free synthesis and characterization of monodisperse magnetite hollow
nanoparticles through solvothermal process. J Nanosci Nanotechnol. 13(8):5773-5776.
2. Nguyen D T, Park D W, Kim T and Kim K S (2013) Controlled synthesis of magnetite porous/hollow nanoparticles
through a template-free solvothermal process. J Nanosci Nanotechnol. 15(1):591-594.
3. Nguyen D T and Kim K S (2013) Analysis on development of magnetite hollow spheres through one-pot solvothermal
process. AIChEJ. 59(10):3594-3600.
Kyo-Seon Kim et al., J Biotechnol Biomater 2018, Volume 8
DOI: 10.4172/2155-952X-C8-109


















