
Page 53
conferenceseries
.com
Volume 5, Issue 3 (Suppl)
Mod Chem Appl, an open access journal
ISSN: 2329-6798
Global Chemistry 2017
September 04-06, 2017
September 04-06, 2017 | London, UK
5
th
Global Chemistry Congress
Synthesis, spectroscopic and structural analysis with electron density distribution of (-)-cytisine and
its derivatives
Anna K Przybył, Agata Owczarzak, Dominika Gołowin
and
Maciej Kubicki
Adam Mickiewicz University in Poznań, Poland
Statement of the Problem:
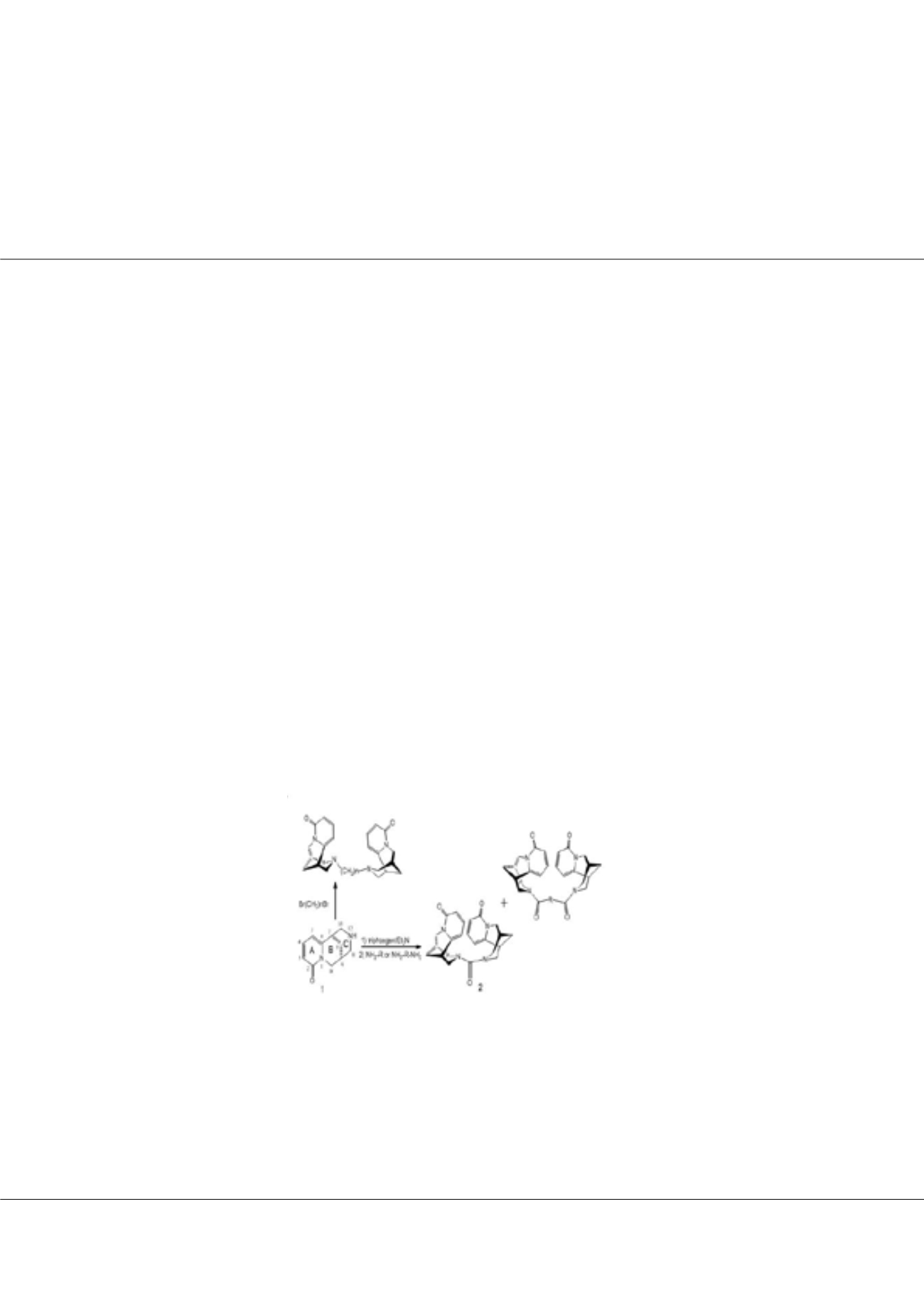
(-)-Cytisine (1, Scheme 1) is an alkaloid, naturally occurring in plants of the Leguminosae family.
It interacts with nAChRs and has been applied in investigation of the central nervous system. Moreover, cytisine derivatives
have been tested for their use in the treatment of Alzheimer’s and Parkinson’s diseases. This alkaloid has been found to
moderately increase the concentration of dopamine alleviating the symptoms of nicotine deprivation. Therefore, cytisine
and its derivative (varenicline) have been employed in nicotine withdrawal therapy. Some N-derivatives of (-)-cytisine show
analgesic, antidepressant, anti-inflammatory, hypoglycemic and hypertension reducing properties.
Methodology&Theoretical Orientation:
(-)-Cytisine was isolated from the seeds of
Laburnumanagyroides
.Themodifications
were made with alkane dibromide or BTC/amines/diamines. The products were separated by flash chromatography. Structural
data were collected using Rigaku diffractometers: Supernova with Atlas detector and Excalibur E. The NMR spectra were
measured on NMR Bruker Advance II 600 MHz.
Findings:
Biological activity of (-)-cytisine is related to the presence of pyridone ring A and piperidine ring C. Such structure
also permits obtaining a number of derivatives with biological activity even greater than that of cytisine itself. It has been found
that modifications of the molecular structure change the pharmacological properties including the affinity to certain nAChR
subtypes. We decided to use the high-resolution X-ray diffraction method which allows for experimental determination of the
details of the electron density distribution in molecular crystals and brings the details of e.g. electrostatic potential and dipole
moments that are the features underlying the biological action.
Conclusion & Significance:
(-)-Cytisine is an excellent substrate in syntheses of the adducts in which other molecules can be
bonded to cytisine through N-12 of piperidine ring C (Scheme 1). The hybrid compounds of this type often show much better
therapeutic properties than the parent ones. Additionally basing on the relation of the electronic/energetic features with the
biological activity, it should be in future possible to design more potent compounds and even to build the pharmacophore
model.
Biography
Anna K Przybył completed her Graduation in Chemistry at Adam Mickiewicz University in Poznań, Poland. In 2000, she received her PhD degree from the same
institution under the supervision of Prof. Waleria Wysocka. She was a Post-doctoral fellow at National Institutes of Health, NIDDK, Laboratory of Medicinal
Chemistry, Bethesda, MD, USA (2001–2003), under the supervision of Dr. Kenner C Rice. In 2015, she obtained her Habilitation degree. She is currently an
Associate Professor at Adam Mickiewicz University, Poznań. Her scientific interests include natural products, especially alkaloids, modern organic synthesis and
medicinal chemistry. Her research focuses on modification of quinolizidine alkaloids, in particular transformations into conjugated systems, enantiomer separation
and structure analysis.
annaprz@amu.edu.plAnna K Przybył et al., Mod Chem Appl 2017, 5:3(Suppl)
DOI: 10.4172/2329-6798-C1-006
















