
Page 68
conferenceseries
.com
Volume 7, Issue 3 (Suppl)
J Obes Weight Loss Ther, an open access journal
ISSN: 2165-7904
Childhood Obesity & Bariatric Surgery 2017
June 12-13, 2017
June 12-13, 2017 Rome, Italy
&
Childhood Obesity and Nutrition
10
th
International Conference on
Metabolic and Bariatric Surgery
2
nd
International Conference on
JOINT EVENT
Maternal obesity leads offspring to alterations in miRNA expression and metabolic phenotype and
negatively impacts in glucose and lipid homeostasis of F2 generation
Laís Simino, Mancini M, Panzarin C, Fontana M F, Milanski M, Torsoni M A, Lgnacio-Souza L M
and
Torsoni A S
State University of Campinas, Brazil
Background & Aim:
Changes in nutritional status in embryonic development and lactation period, as an excessive caloric intake,
may lead to a phenomenon known as metabolic programming. Moreover, recent studies have shown that maternal obesity can
have transgenerational effects, affecting not only the F1 generation, but also future generations. These effects, transmitted across
generations, can be triggered by epigenetics mechanisms, such as miRNA expression. The miRNA Let-7 is shown to be involved in
glucose homeostasis and here, we aimed to evaluate its expression in the liver of F1 offspring from obese mothers and the impacts
of its modulation to the F2 generation.
Methods:
Female Swiss mice were fed with a HF or control diet for an adaptation period and through gestation and lactation. Weaned
offspring received control diet until d28. Part of female offspring remained in control diet until mating to generate F2 offspring, which
were weaned and received control diet until d28.
Results:
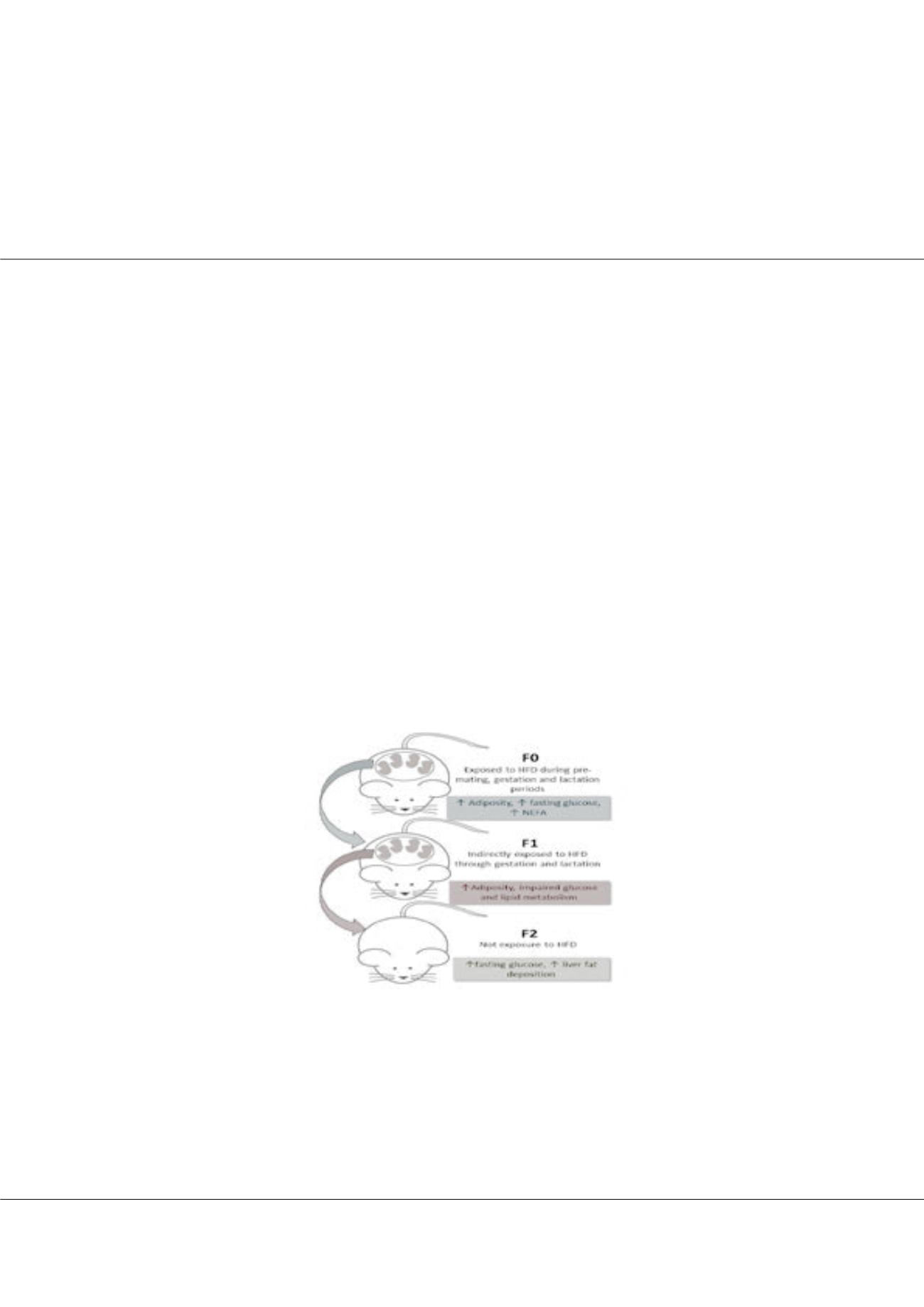
After the adaptation period, F0 females that consumed a HFD were divided in two groups: Obese prone (OP) or obese
resistant (OR), according to their weight gain. OP presented higher body weight, adiposity, serum glucose and NEFA than OR. Male
and female offspring fromOR and OP (OR-O and OP-O) showed an increase in body weight and adiposity at d28, but OP-O presented
impaired glucose tolerance and insulin sensitivity, besides higher serum lipid biomarkers. F1 OP-O also had an overexpression in
hepatic Let-7 and down-regulation of AMPK, a predicted mRNA target of this miRNA. F2 offspring showed no alteration in body
weight and adiposity, but F2 OP-O presented higher fasting glucose as early as d0 and d28, and an elevated liver fat content.
Conclusion:
Nutritional overload in critical periods of development leads offspring to epigenetic changes that may have
transgenerational negative impacts.
Figure 1:
Summary of the experimental design and results found in the present study.
Biography
Laís Simino is a Nutritionist and pursuing her PhD at State University of Campinas – UNICAMP. She belongs to the Obesity and Comorbidities Research Center
(OCRC) and Laboratory of Metabolic Diseases, a laboratory that has been specializing in fetal programming research, especially triggered by maternal consumption
of high fat diets.
lais.simino@fca.unicamp.brLaís Simino et al., J Obes Weight Loss Ther 2017, 7:3 (Suppl)
DOI: 10.4172/2165-7904-C1-046
















