
Volume 7, Issue 5(Suppl)
J Bioremed Biodeg 2016
ISSN: 2155-6199 JBRBD, an open access journal
Page 14
Biopolymers and Bioplastics 2016
September 12-14, 2016
conference
series
.com
September 12-14, 2016 San Antonio, USA
3
rd
International Conference and Exhibition on
Biopolymers & Bioplastics
Richard A Gross, J Bioremed Biodeg 2016, 7:5(Suppl)
http://dx.doi.org/10.4172/2155-6199.C1.001Structure property relationships of biobased epoxy resins
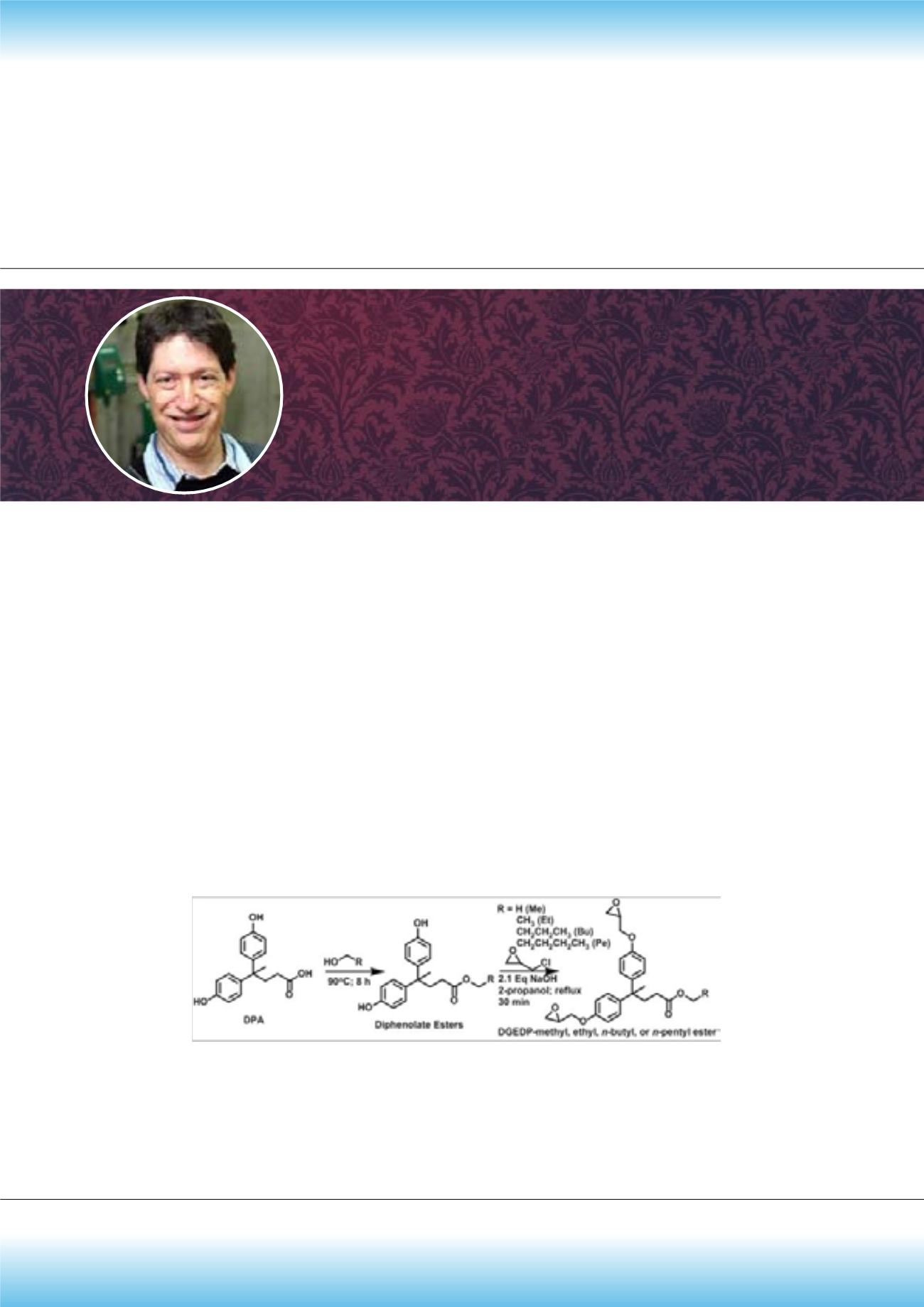
L
evulinic acid is produced from cellulose, the most abundant biomacromolecule on the planet, by the acid hydrolysis
of cellulose and resulting C
6
sugars. Diphenolic acid (DPA) is synthesized by condensation of levulinic acid with two
equivalents of phenol. A series of bio-based epoxy monomers were prepared from diphenolic acid (DPA) by transforming the
free acid into n-alkyl esters and the phenolic hydroxyl groups into diglycidyl ethers. Increasing the chain length of DGEDP
n-alkyl esters frommethyl to n-pentyl resulted in large decreases in epoxy resin viscosity (700-to-11 Pa.s). The storage modulus
of DPA epoxy resins, cured with isophorone diamine, also varied with n-alkyl ester chain length (e.g. 3300 and 2100 MPa for
the methyl and n-pentyl esters). The Young’s modulus and tensile strengths were about 1,150 and 40 MPa, respectively, for all
the cured resins tested (including DGEBA) and varied little as a function of ester length. This work demonstrates that diglycidyl
ethers of n-alkyl diphenolates represent a new family of bio-based liquid epoxy resins that, when cured, have similar properties
to those from DGEBA. Combinations of DGEDP-Me, a rigid high viscosity biobased epoxy resin, and a flexible lower viscosity
epoxy resin from cashew nut shell liquid (NC-514), provided control of the resin viscosity and important improvements in
cured epoxy resin toughness relative to the neat resins. Relative to the neat high viscosity resin, 1:1 w/w mixtures of the rigid
and flexible epoxy resin components gave increases in the impact strength and mode I fracture toughness of 136% and 66%,
respectively. The monofunctional glycidyl ether of eugenol (GE) was used as a reactive diluent for the diglycidyl ether of
DGEDP-Pe. Viscosities of GE and DGEDP-Pe are 25 MPa.s and 11 Pa.s, respectively. GE/DGEDP-Pe epoxy resins with 5, 10,
15, 20, and 30 wt % GE were analyzed for viscosity reductions, and, subsequently, cured with isophorone diamine. The glassy
modulus of cured GE/DGEDP-Pe epoxy resins remained between 2000 and 3000 MPa. The role of GE as a reactive diluent
will be discussed and a 15% loading was determined to be suitable for a vacuum infusion epoxy resin/glass composite system.
Biography
Richard A Gross is currently a Full Professor and a Constellation Chaired Professor at Rensselaer Polytechnic Institute (RPI). His research is focused on
developing biocatalytic routes to biobased materials including monomers, macromers, prepolymers, polymers, surfactants and other biochemicals. He has over
500 publications in peer reviewed journals, been cited about 18,000 times (h-index 71), edited 7 books and has 26 patents (granted or filed). He was the recipient
of the 2003 Presidential Green Chemistry Award in the academic category. In 2010, he was selected as the Turner Alfrey Visiting Professor, and in 2015 he became
a Fellow of the ACS Polymer Division. He founded SyntheZyme LLC in 2009 and serves as CTO.
grossr@rpi.eduRichard A Gross
Rensselaer Polytechnic Institute, USA
















