Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Google Scholar citation report
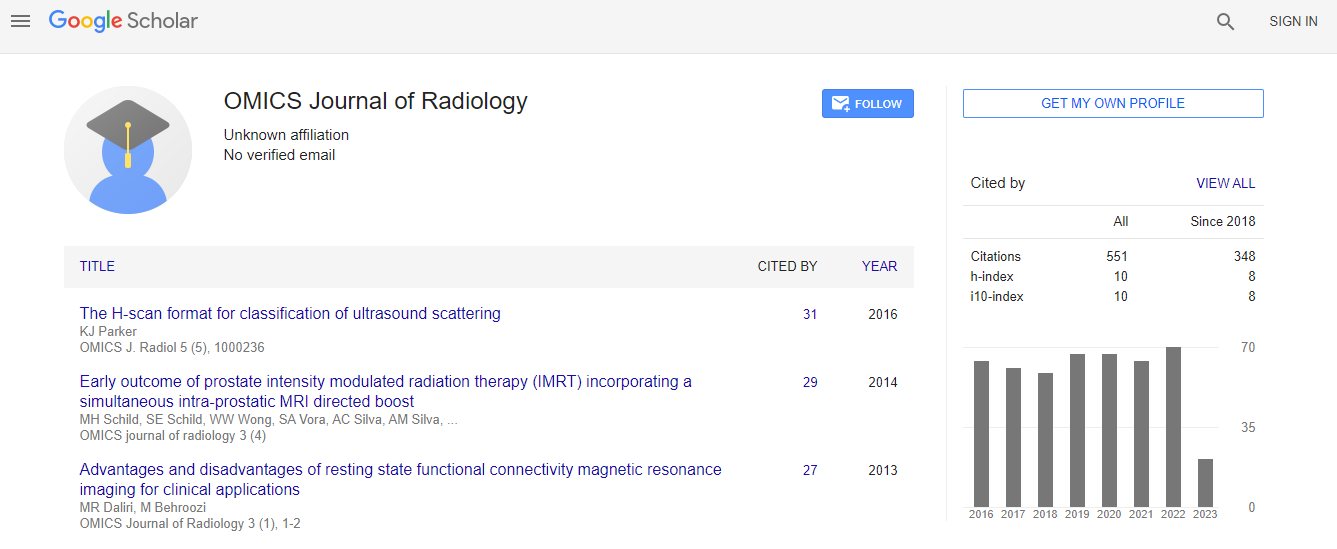
Citations : 551
Journal of Radiology received 551 citations as per Google Scholar report
Journal of Radiology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- ICMJE
Useful Links
Share This Page
Why oncology field need Biosimilars?
2nd World Congress on Radiology and Oncology
Abdalla Abotaleb
World Health Organization, Egypt
ScientificTracks Abstracts: OMICS J Radiol
Abstract
Background: One of the major products in treatment expenditures at oncology field are biological products. Representing a remarkable percentage of oncology pipeline which increase economic burden on payers and may lead to treatment restrictions due to high cost of biologicals. Introducing biosimilars products may offer safe, effective, sometimes cost saving alternative to innovator biological therapies, which may lead to change treatment polices due to different alternatives represented by biosimilars. Objective: The main objective is to evaluate introducing biosimilars to the oncology treatment through guidelines modification, numbers of treated patients, price discounts for innovator products and quality of service introduced to the patients. Method: Data analyzed for (113,429) cancer patient for the last 3 years from national database including (treatment guidelines-patients satisfaction serves-reimbursement lists-price offers for innovator). Local biosimilars guidelines were the reference for estimating local biosimilars. Result: Introducing biosimilars products to Egyptian market at last 3 years lead to changing neutropenia guidelines were modified for including (GCSF as a routine treatment for both prophylactic and after chemotherapy). HER2+ guidelines modified to contain monoclonal products as a standard of care for both adjuvant and metastatic cases. Monoclonal antibodies were included at NHL guidelines. Number of treated cancer patients increased by 40% last 3 years. Price discounts for innovator products were found in values ranged from 35%-66%. Surveys illustrated that patient’s satisfaction about introducing new products reducing time of treatment for neutropenia patients, hospitalization time decreased due to modification of neutropenia guidelines. Conclusion: Introducing biosimilars to the oncology field may lead to offer safe, effective efficient solution for controlling budget and enhancing health service. Biosimilars may have a major role for achieving perfect computation at oncology field.Biography
Abdalla Abotaleb, MD is a World Health Organization expert. He is also a consultant on health economics at the Egyptian Ministry of Health, as well as a member of the Egyptian health care reforming committee Ispor (member, judge and reviewer).
E-mail: thepharmacist7777@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi