Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
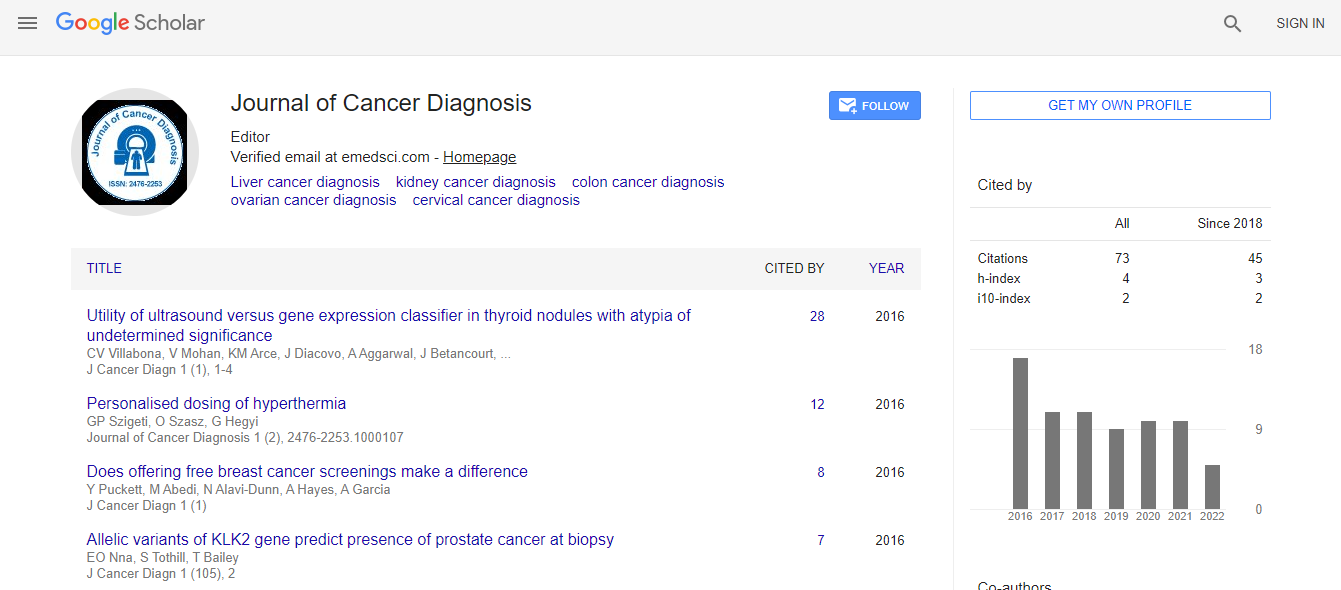
Google Scholar citation report
Citations : 95
Journal of Cancer Diagnosis received 95 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
U.S. Biosimilars Market - A Deep Dive
18th Global Biomarkers and Clinical Research Summit
Aiswariya Chidambaram
Associate Director, USA
ScientificTracks Abstracts: J Cancer Diagn
Abstract
Ever since the approval of the first biosimilar drug in the U.S. in 2015, 31 biosimilars have been approved till date (as of September 2021). While 2019 witnessed a steep surge in the approval rates of biosimilars, the COVID-19 pandemic and the resulting lockdown did slow down the approval rates over the last one year (2020 ΓΆΒ?Β? 2021). Nevertheless, the U.S. biosimilars market is poised to witness a significant growth of 25% (CAGR) over the next seven years (2021 ΓΆΒ?Β? 2028) propelled by the patent expiry of key blockbuster drugs, increased cost-saving initiatives and conducive regulatory framework established by the U.S. government, strategic partnerships, and industry consolidation in addition to the rising incidence of chronic diseases and aging baby-boomer population. Both the biotech firms as well as regulatory authorities have emerged resilient and geared well to a post-pandemic world. This market research service analyses the revenue prospects of the U.S. biosimilars market (in terms of market size, growth rates, YOY revenue forecasts, etc.), patent cliff (biologic drugs due to lose patent protection), key therapeutic areas overview, competitive landscape, key strategic alliances, regulatory framework, pricing and reimbursement policies as well as impact mapping of patients, physicians and payers. Furthermore, strategic recommendations for the success of market participants have also been discussed.Biography
Aiswariya Chidambaram is a management consultant project leader with over 11 years of experience in the Life Sciences (Pharmaceutical & Biotechnology) sector. To her credits, Aiswariya has Consulted and provided strategic insights to several leading multinational pharmaceutical and biotech firms across the globe. Authored over fifteen research reports on key therapeutic and service areas for the global pharmaceutical and biotechnology markets. Authored over thirty publications including whitepapers, ad-hoc articles and expert interviews in reputed magazines and journals. Been a plenary/key-note speaker at reputed industry conferences including CPhI, Pharma Tech, United Pharma, among others. Identified and acknowledged companies demonstrating excellence in specific market/product segments and technologies as part of the industry best practices research.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi