Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
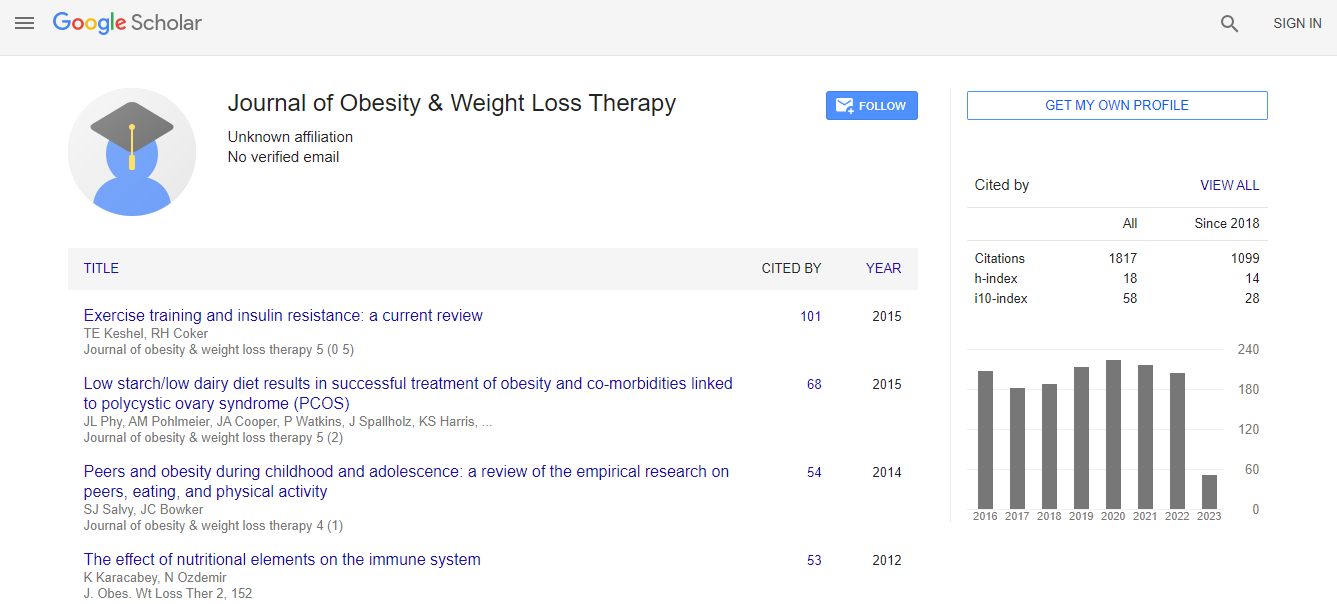
Google Scholar citation report
Citations : 2305
Journal of Obesity & Weight Loss Therapy received 2305 citations as per Google Scholar report
Journal of Obesity & Weight Loss Therapy peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- CABI full text
- Cab direct
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- University of Bristol
- Pubmed
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Unraveling the role of gut hormone PYY in diabetes remission and pancreatic islet function following Roux-En-Y gastric bypass
JOINT EVENT 10th International Conference on Childhood Obesity and Nutrition & 2nd International Conference on Metabolic and Bariatric Surgery
Reshma Ramracheya, Anne Clark, Helene Johannessen, Magnus Kringstad Olsen, Chun-Mei Zhao, Duan Chen and Patrik Rorsman
University of Oxford, UK
ScientificTracks Abstracts: J Obes Weight Loss Ther
Abstract
Roux-En-Y gastric bypass (RYGB) results in long-lasting remission from type-2 diabetes (T2D) in most cases. Improvements in glucose homeostasis occur within days of surgery, but the mechanisms involved remain unclear. Although pancreatic islets play a fundamental role in glucose homeostasis, the impact of RYGB on islet architecture and secretory properties has not been studied thoroughly. T2D is a bihormonal disease characterized by both insufficient insulin secretion and impairment in glucagon regulation. RYGB can correct both hormonal secretory defects remain unexplored. Using the Goto-Kakizaki (GK) rat model of T2D, we have explored whether RYGB affects islet structure and glucose-stimulated insulin secretion (GSIS) and glucagon release. RYGB restored distorted islets from diabetic rats to spheroidal shape as in healthy animals. Compared to the sham-operated animals, RYGB normalized glucose-dependent glucagon and insulin secretion. Thus, islets from RYGB rats exhibited markedly enhanced insulin secretion at 20 mM glucose and complete restoration of glucose-induced suppression of glucagon release. Culture of isolated islets with serum from RYGB animals resulted in improved insulin and glucagon secretion, in support of a humoral factor which remains conserved in serum. These effects were reversed following immuno-neutralization of the gut hormone peptide tyrosine (PYY) but persisted in the presence of a glucagon-like peptide-1 (GLP-1) receptor antagonist. Chronic (60-72 h) treatment of islets with synthetic PYY enhanced GSIS in a NPY1-receptor-dependent manner. PYY application also restored GSIS and normalized impaired glucose-induced glucagon release in islets from severely diabetic GK rats and human donors with T2D. These data provide a novel mechanistic insight on the effect of RYGB in diabetes and highlight that the mechanism behind the improvement of islet function is dependent on PYY and not GLP-1. These findings imply that a pharmacological agent enhancing PYY release or its action could provide an effective and non-surgical therapy for T2D.Biography
Reshma Ramracheya is investigating the regulation and failure of the insulin-secreting beta-cell and glucagon-secreting alpha-cell in normal health and diabetes respectively. As an islet Physiologist, she intrigued by how pancreatic islet cells, despite being biochemically similar, are able to respond to nutrients and drugs differently.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi