Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
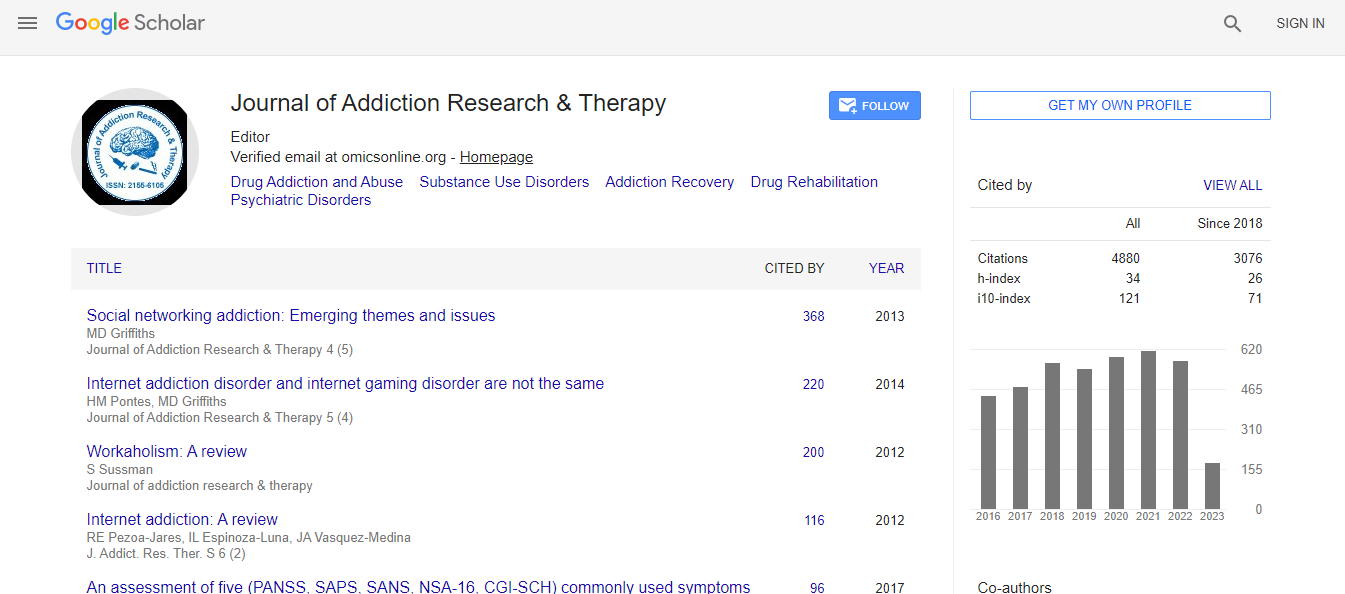
Google Scholar citation report
Citations : 4859
Journal of Addiction Research & Therapy received 4859 citations as per Google Scholar report
Journal of Addiction Research & Therapy peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- SafetyLit
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Three dimensional structure of Human nicotinic acetylcholine receptor ���±7 constructed through Homology modelling and Molecular dynamics simulation
4th International Conference and Exhibition on Addiction Research & Therapy
Huixiao Hong1, Hui Wen Ng1, Michael Orr2 and Weida Tong1
ScientificTracks Abstracts: J Addict Res Ther
Abstract
Addiction to nicotine, and possibly other tobacco constituents, is a major factor that contributes to the difficulties smokers face when attempting to quit smoking. Amongst the various subtypes of nicotinic acetylcholine receptors (nAChRs), the ���±7 subtype plays an important role in mediating the addiction process. The human nAChR ���±7 is prevalent in the central nervous system, particularly in the hippocampus area of the brain, and is deemed as a promising target for smoking cessation therapies, treatment of neuropsychiatric and inflammatory disorders, amongst others. To date, a human structure of the nAChR ���±7 has not been elucidated. A homodimeric homology model of the extracellular ligand-binding domain of human nAChR I�±7 was constructed based on the crystal structure of the epibatidine-bound human nAChR ���±7 and Lymnaea stagnalis acetylcholine binding protein (AChBP) chimera protein (PDB ID 3SQ6), which share 71% similarity with the native human nAChR ���±7. With the cognate ligand preserved in the binding pocket, a 100 ns molecular dynamics (MD) simulation was conducted to refine the homology model. The RMSD plot from the resultant trajectory shows that the protein achieved a steady state after ~20ns simulation with a stable fluctuation of approximately 3 while the ligand after ~35ns with a stable fluctuation of <0.5. The refined structure could assist in identifying tobacco constituents that may have human I�±7 nAChR binding activity.Biography
Huixiao Hong received Ph.D. in computational chemistry at Nanjing University, China in 1990 and completed postdoctoral fellowship at the Maxwell Institute at Leeds University in UK in 1992. He is a Sr. Scientist at the US FDA. He has published more than 130 papers in reputed journals and has been serving as an editorial board member of repute.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi