Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
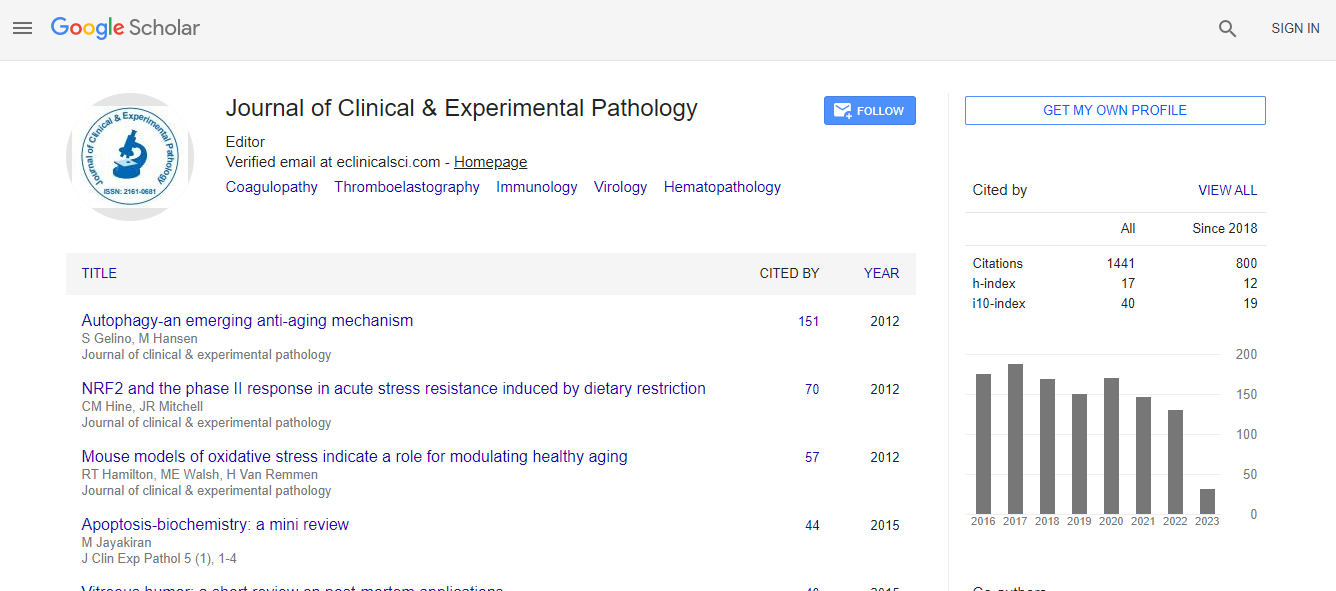
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
Theranostics in cytopathology to address the need for personalized medicine in healthcare
14th Asia Pacific Pathology Congress
E Blair Holladay
American Society for Clinical Pathology, USA
Keynote: J Clin Exp Pathol
Abstract
The field of cytopathology has evolved from basic Pap staining of tumors followed by H&E tissue diagnosis of disease to the use of immunocytochemistry and complementary ancillary molecular diagnostics to aid in specifying the disease. However, due to the sequencing of the human genome and the subsequent genomic revolution, the field of theranostics has evolved. Theranostics is the coupling of companion diagnostic tools (in particular, molecular profiling) with specific therapeutic drugs. This "personalized" approach to diagnosis allows the clinician to provide therapy based on specific genetic mutations of the tumors from their patients. The FDA has dramatically increased the number of cleared/approved in vitro assays for patients with genetic mutations that respond to drugs that prevent the expression of the mutations, such as tyrosine kinase inhibitors. These alternative forms of therapy have dramatically increased the survival rate in patients with stage four and metastatic cancer. It is imperative that pathologists and laboratory professionals determine which companion diagnostic assay should be chosen and recommend the clinically actionable drugs tailored to their genetic mutation to the clinician. This change in the scope of practice creates unprecedented opportunities to more accurately diagnose patients and guide the selection of personalized therapies.Biography
E Blair Holladay is the Chief Executive Officer of the American Society for Clinical Pathology (ASCP), USA. He has focused on globalization initiatives for the medical laboratory community that include significant contributions to the President’s Emergency Plan for AIDS Relief (PEPFAR) funded through the Centers for Disease Control and Prevention; strategic partnerships in laboratory medicine; corporate reorganization and management activities; mergers and acquisitions; international outreach; external partnerships; health services research and delivery and also establishing the gold standard of certification for individuals within the United States and worldwide.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi