Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaGoogle Scholar citation report
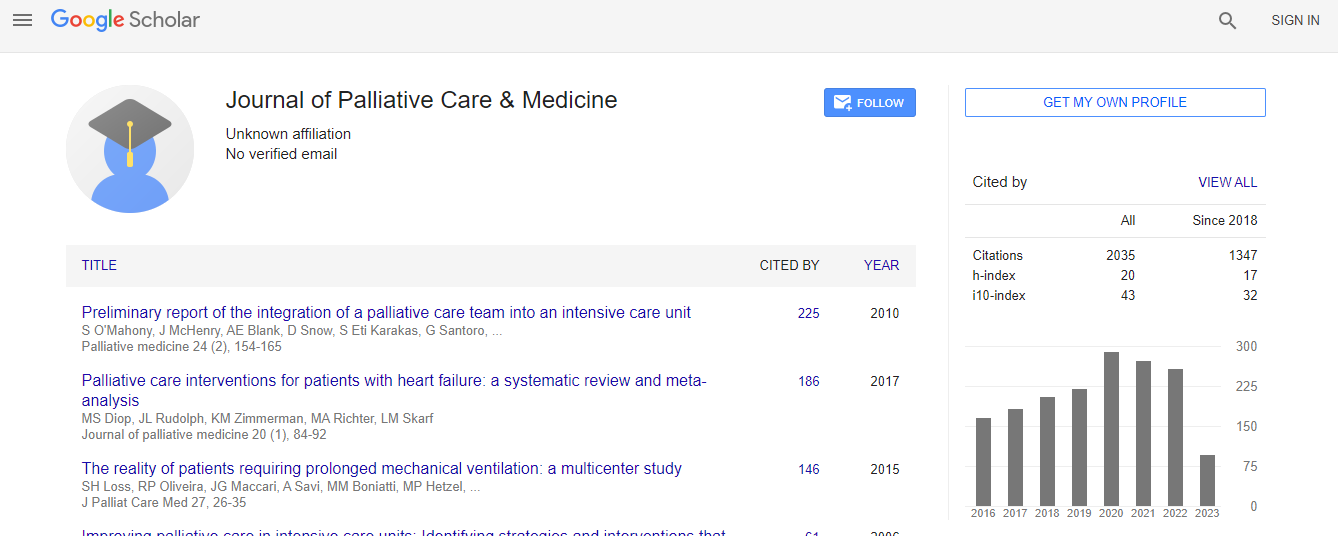
Citations : 2035
Journal of Palliative Care & Medicine received 2035 citations as per Google Scholar report
Journal of Palliative Care & Medicine peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
THE SYSTEMATIC EARLY INTEGRATION OF PALLIATIVE CARE INTO MULTIDISCIPLINARY ONCOLOGY CARE IN THE HOSPITAL SETTING (IPAC), A RANDOMIZED CONTROLLED TRIAL: THE STUDY PROTOCOL
2nd Global Congress on Hospice & Palliative Care
Gaelle Vanbutsele, Simon Van Belle, Martine De Laat, Veerle Surmont, Karen Geboes, Kim Eecloo, Keon Pardon and Luc Deliens
Ghent University, Belgium
Posters & Accepted Abstracts: J Palliat Care Med
Abstract
Background: Previous studies in the US and Canada show the positive impact of early palliative care programs for advanced cancer patients on quality of life (QoL) and even survival time. There has been a lack of similar research in Europe. In order to generalize the findings from the US and Canada research on a larger scale, similar studies are needed in different countries with different care settings, such as Belgium. Method: A randomized controlled trial (RCT) is being conducted as follows: 186 patients with advanced cancer were recruited from the departments of Medical Oncology, Digestive Oncology and Thoracic Oncology of the Ghent University Hospital. Patients are randomized to either systematic early integration of palliative care in standard oncology care or standard oncology care alone. Patients and informal caregivers are asked to fill out questionnaires on QoL, mood, illness understanding and satisfaction with care at baseline, 12 weeks and every six weeks thereafter. Other outcome measures are end-of-life care decisions and overall survival time. Results concerning baseline characteristics are due June 2016. Discussion: This is the first RCT in the Belgian health care setting to evaluate the effect of systematic early integration of palliative care for advanced cancer patients. The results will enable us to evaluate whether systematic early integration of palliative care has positive effects on QoL, mood and patient illness- Understanding and which components of the intervention contribute to these effects. Trial registration Clinical trials.gov Identifier: NCT01865396, registered 24th of May, 2013.Biography
Email: gaelle.vanbutsele@uzgent.be

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi