Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
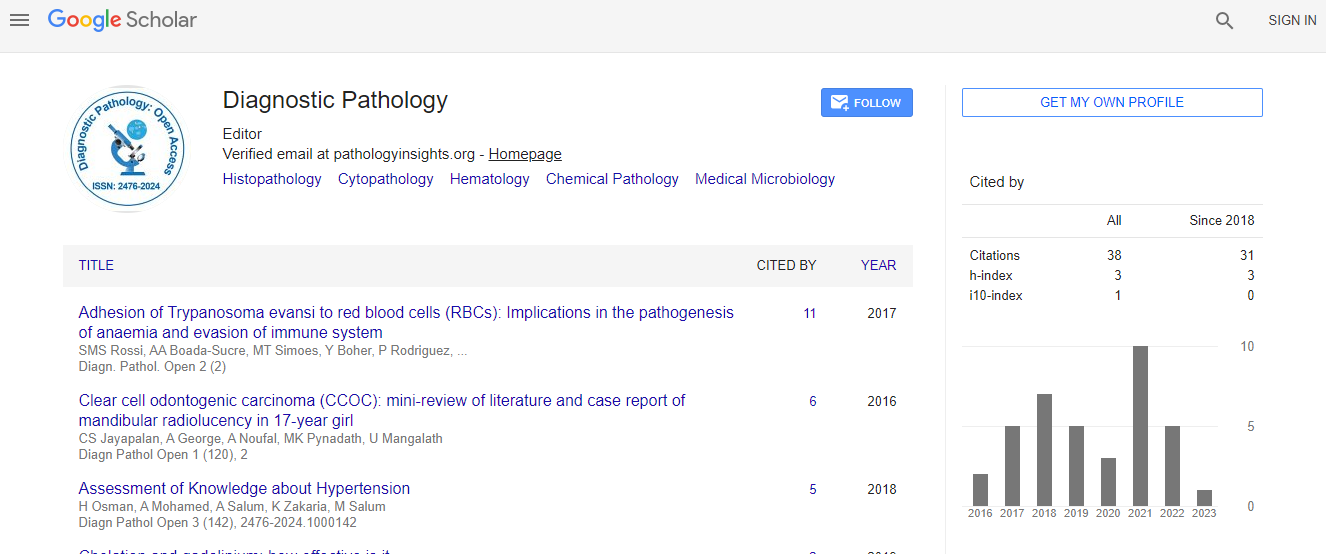
Google Scholar citation report
Citations : 110
Diagnostic Pathology: Open Access received 110 citations as per Google Scholar report
Diagnostic Pathology: Open Access peer review process verified at publons
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Share This Page
The Periplasmic Chaperone Network Of Campylobacter Jejuni: Evidence That SalC (Cj1289) and PpiD (Cj0694) Are Involved In Maintaining Outer Membrane Integrity
13th International Conference on Laboratory Medicine & Pathology
Shadi A Zakai, Aidan J Taylor and David J Kell
King Abdulaziz University, Saudi Arabia
Posters & Accepted Abstracts: Diagn Pathol Open
Abstract
The outer membrane (OM) of Gram-negative pathogenic bacteria is a key structure in host-pathogen interactions that contains a plethora of proteins, performing a range of functions including adhesion, nutrient uptake, and antimicrobial resistance. The OM of the food-borne pathogen Campylobacter jejuni contains crucial proteins that do this function, and must be specifically localized to this membrane, but the sorting mechanisms involved are only partially understood. In particular, chaperones are required to ferry OM proteins across the periplasm after they emerge from the Sec translocation system. In this work, we have constructed a set of isogenic deletion mutants in genes encoding both known and predicted chaperones (cj0596, cj0694, cj1069, cj1228c, and cj1289) using NCTC 11168H as the parental strain. These mutants were characterized using a range of assays to determine effects on growth, agglutination, biofilm formation, membrane permeability and hydrophobicity. We focused on Cj1289 and Cj0694, which our previous work suggested possessed both chaperone and peptidyl-proyl cis/trans isomerase (PPIase) domains. Mutants in either cj1289 or cj0694 showed growth defects, increased motility, agglutination and biofilm formation and severe OM permeability defects. 2D-gel comparisons showed a general decrease in OM proteins in these mutants. We heterologously overproduced and purified Cj0694 and obtained evidence that this protein was an active PPIase, as it accelerates the refolding rate of reduced and alkylated ribonuclease T1 and that it also possessed holdase-type chaperone activity. Cj0694 is most similar to the PpiD class of chaperones but is unusual in possessing PPIase activity. Our data show that Cj1289 (SalC; SurA-like chaperone) and Cj0694 (PpiD) are key proteins involved in OM biogenesis and integrity in C. jejuni.Biography
Shadi A Zakai has completed his PhD from the University of Sheffield, UK. He currently works in the Department of Medical Microbiology and Parasitology, Faculty of Medicine, King Abdulaziz University, Kingdom of Saudi Arabia. He has published 4 papers in reputed journals and has been serving as an Editorial Board Member of repute journals. His research interest include: clinical microbiology, molecular biology, bacterial resistance to antimicrobial agents, and infection prevention and control.
E-mail: szakai@kau.edu.sa

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi