Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
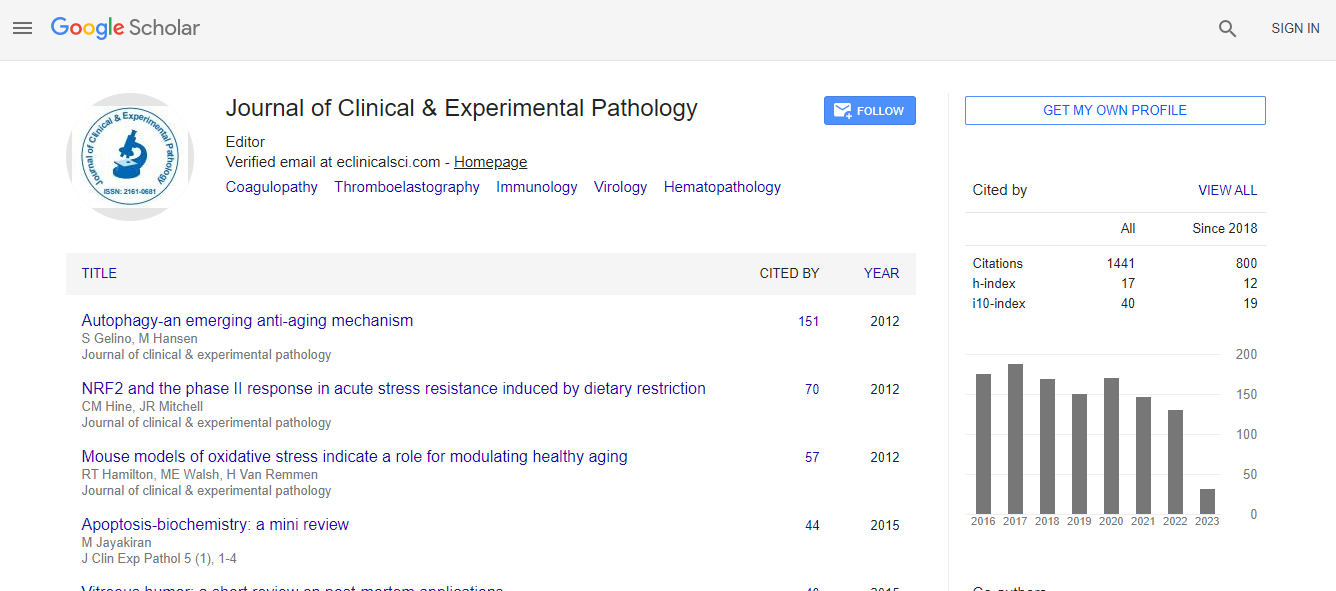
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
The new trend in laboratory assay of NOACs
15th Global Experts Meeting on Pathology and Laboratory Medicine
Sin Chun Fung
University of Hong Kong, China
ScientificTracks Abstracts: J Clin Exp Pathol
Abstract
New Oral Anti-Coagulants (NOACs) are getting more and more popular now and vast majority of patients are taking NOACs as thrombo-prophylaxis now. The merits of NOACs include wide therapeutic index and stable pharmacokinetics and hence there is no need for laboratory monitoring. However, there are some clinical situations that laboratory monitoring of NOACs is important for patient├ó┬?┬?s management; for example, patients undergoing invasive procedures or patients suffering from bleeding complications. Various platforms of laboratory assay are available for measuring the drug level of dabigatran, rivaroxaban and apixaban. The measurement of rivaroxaban and apixaban can be done by anti-Xa assay and dilute thrombin time can be used to measure the level of dabigatran. Some review articles mentioned that the value of specific assay of NOACs is uncertain, mainly because the precision and accuracy of the specific assay is not optimal, especially for low drug level. Nowadays, some companies provide kit with low-level calibrators to improve precision of measurement of low drug level. In our study, we use the anti-Xa kits and dilute thrombin time kits from Werfen Company and Sysmex Company for evaluating the drug assay of dabigatran and rivaroxaban. The precision, accuracy, linearity and limit of detection are satisfactory for measuring various levels of dabigatran and rivaroxaban, including low drug concentrations and the performance of the kits provided by two companies are comparable. The relationship between the drug levels of NOACs with routine coagulation screening tests was also evaluated. The issue of quality control and some practical issues for implementation of the specific laboratory assay of NOACs will also be discussed.Biography
Sin Chun Fung has obtained his Medical degree in 2008 and is a qualified Hematopathologist in Hong Kong. Currently he is working as a Clinical Assistant Professor in University of Hong Kong. He had been working as Physician in the past. He is interested in the work and research in various hematological malignancies and investigating the novel treatments. He is also passionate in the field of coagulating and hemostasis. With his experience and expertise in internal medicine, he had done work on the laboratory assay of new oral anti-coagulants, with the aim of improving the management of patients taking NOACs.
E-mail: scf185@pathology.hku.hk

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi