Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
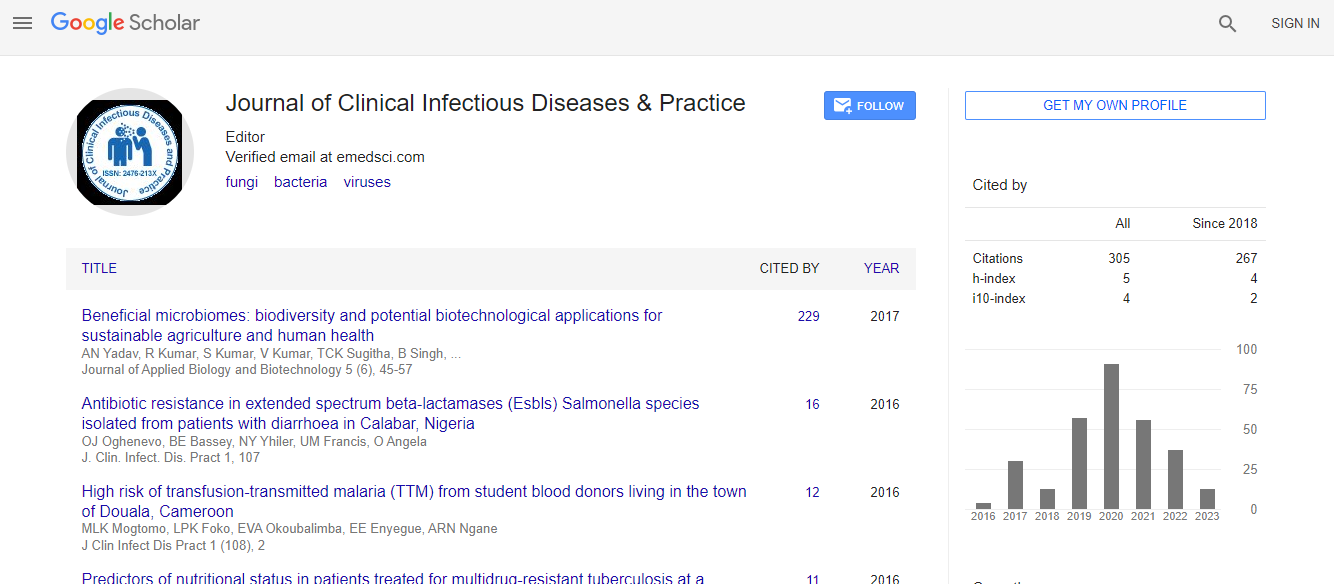
Google Scholar citation report
Citations : 531
Journal of Clinical Infectious Diseases & Practice peer review process verified at publons
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Share This Page
The Economics and Sustainability of Orphan Drugs
9th World Congress on Rare Diseases and Orphan Drugs
Carina Schulmann Schey
University of Groningen, Netherlands
ScientificTracks Abstracts: J Clin Infect Dis Pract
Abstract
Orphan drugs are increasingly under scrutiny by reimbursement bodies in Europe. This is due in part to the unexpected rise in the number of orphan drugs that received marketing authorization since 2000. The high prices of some orphan drugs and the budget constraints, affordability and sustainability of access to orphan drugs further contribute to the sometimes- negative spotlight on orphan drugs. However, one of the limitations in the current reimbursement pathways is the use of cost-effectiveness analyses to assist in the decision-making process. Increasingly, payers and policy makers highlight the need for alternative methods of assessing the value of orphan drugs and demonstrating their ongoing accessibility. A novel approach, using multi-criteria decision analysis, was developed to review orphan drugs. The framework has been tested with useful results. The strength and versatility of multi-criteria decision analysis is that it permits the different criteria to be assigned different weight based on their relevance to the hypothesis being tested. But one of the limitations is the lack of experience in the weighting of the criteria. In pursuance of in-depth insights on the weight that should be allocated to each criterion, an interactive web-based tool was developed that allowed respondents to complete by allocating the weights they thought suitable for each criterion. This presentation will provide the economic background in the provision of treatments for rare diseases, share some of the key economic and financial hurdles and provide the outcomes of the web-based tool and the perspectives gathered therefrom as well as suggest actions going forward in a bid to improve access to rare disease treatments while ensuring that they remain affordable and therefore ensuring sustainability in the future.Recent Publications
1. Schey C, Milanova T and Hutchings A (2011) Estimating the budget impact of orphan medicines in Europe: 2010-2020. Orphanet Journal of Rare Diseases. 6(1):62.
2. Oliva E N, Schey C and Hutchings A S (2011) A review of anemia as a cardiovascular risk factor in patients with myelodysplastic syndromes. American Journal of Blood Research. 1(2):160-166.
3. Hutchings A, Schey C, Dutton R, Achana F and Antonov K (2014) Estimating the budget impact of orphan drugs in Sweden and France 2013-2020. Orphanet Journal of Rare Diseases., 9(1):22
4. Schey C, Krabbe P F M, Postma M J and Connolly M P (2017) Multi-criteria decision analysis (MCDA): testing a proposed MCDA framework for orphan drugs. Orphanet journal of rare diseases. 12:10.
5. M P Connolly, E Goodwin, C Schey and J Zummo (2017) Toxoplasmic encephalitis relapse rates with pyrimethamine-based therapy: systematic review and meta-analysis. Pathogens and Global Health., 111(1):31- 44.
Biography
Carina Schulmann Schey is pursuing her PhD in the economics of orphan drugs and assessing alternative ways to adjudicate their value-add in the management of rare diseases. With a background as a Clinical Pharmacist with a special interest in rare diseases, she has published several peer-reviewed articles and abstracts in rare diseases. She sits on the expert judges’ panel for the MassChallenge and on the scientific advisory panels for several charities, and as a Non- Executive Director for healthcare organizations.
E-mail: c.f.schey@rug.nl

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi