Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
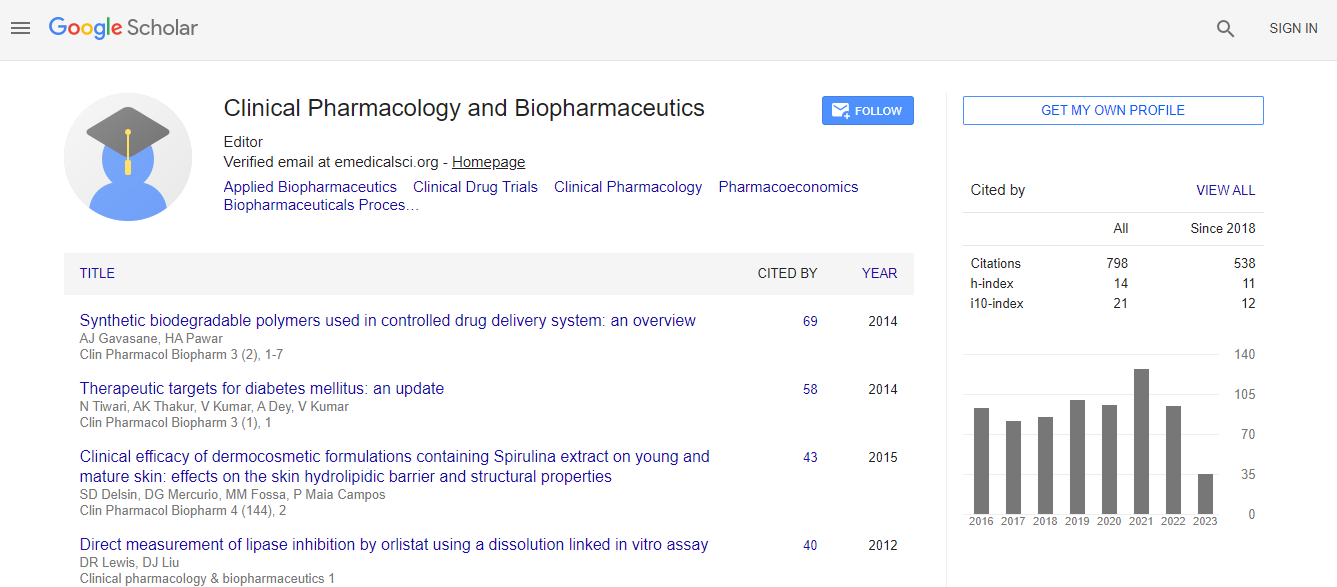
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Targeted vaccination and intrinsic adjuvant function: Next generation checkpoint control of tumor specific B and T cells
Joint Event on 4th European Biopharma Congress & 6th International Conference and Exhibition on Pharmacology and Ethnopharmacology
Geert C Mudde
OncoQR ML GmbH, Austria
Keynote: Clin Pharmacol Biopharm
Abstract
OncoQR develops therapeutic cancer vaccines based on the S-TIRâ�?¢ technology platform. Vaccines from this platform are (non-)human specific and able to induce strong polyclonal B cell and T cell immune responses against tumour associated (auto-)antigens. Two prototype vaccines, TYG100 and OQR200 resp., have reached in vivo proof of concept in non-human primates (NHP). S-TIRâ�?¢ vaccines consist of 2 modules, the disease specific module, â�?�?immunogenâ�? and the generic module, â�?�?warheadâ�?, which directs the vaccines to CD32 on antigen presenting cells, especially pDCs and B cells and optimally activates these cells1. The immunogen of TYG100 is G17, a growth factor for pancreatic cancer cells2 The immunogen of OQR200 targets and contains HER2/neu, overexpressed in ~20% of all breast cancer patients. TYG100 was tested as monotherapy and in combination with gemcitabine. OQR200 were tested as monotherapy and in combination with TYG100 in a cross over study. Four immunizations were given 2-3 weeks apart antibody titres were measured on a weekly basis. Under normal conditions no clinically relevant immune responses can be induced against autoantigens. However, in combination with the warhead, thanks to intrinsic check point control, all treated NHP (n=44) generated very strong and rapid dose dependent auto-antigen specific antibody (IgG and IgA) and T cell responses. Two weeks after the 2nd immunization all animals were seroconverted. Despite very high antibody titres no side effects were observed. Animals, sequentially treated with OQR200, TYG100 and OQR200 showed that the induced responses were 100% vaccine specific, resulting in animals with very high antibody titres against 2 different autoantigens at the same time. All responses are reversible and can be boosted. S-TIRâ�?¢ vaccines do not induce autoimmune disease and are tumour specific while optimally mobilizing both arms of the immune system. The immune response can be fine-tuned on a patient to patient basis.Biography
Geert C Mudde received a PhD in Immunology from the University of Utrecht in 1985 and started his international professional career at the Swiss Institute for Asthma and Allergy Research in Davos in 1989. In 1992, he joined the Pharmaceutical/Biotech Industry, where he held several Senior Management positions at the Novartis Research Institute in Vienna, Austria, the Parke Davis Research Institute in Fresnes, France, Ingenium Pharmaceuticals, Martinsried, Germany, and at Igeneon AG, Vienna, Austria. Finally, in 2006, while joining Baxter BioScience in Vienna as Interim Manager, he co-founded the biotech company F-star Biotechnology. In 2009, together with Christof Langer, he started to develop the S-TIR™ technology platform for human specific therapeutic vaccines which led to the foundation of S-TARget therapeutics GmbH in 2010, and the spin-off companies OncoQR ML GmbH (2013) and TYG oncology Ltd. (2013). He serves as CSO and Managing Director for OncoQR ML.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi