Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
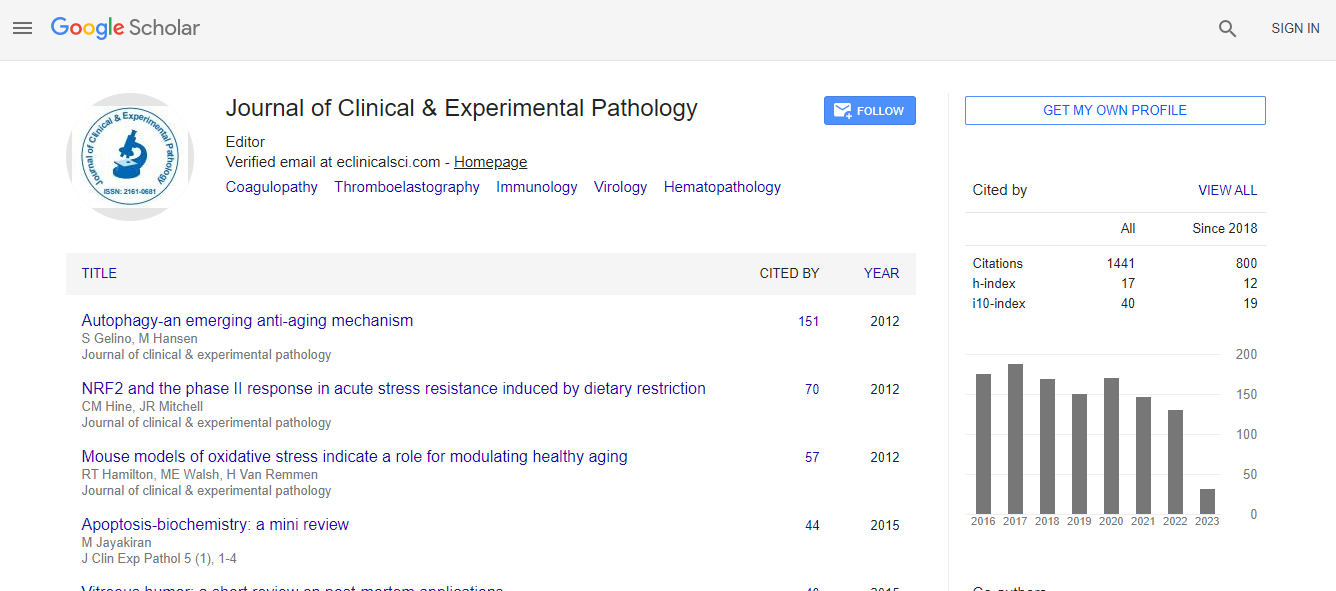
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
Synthesis of azetidines and pyrrolidines: Towards medicinal chemistry and organocatalysis applications
Joint Event on 6th World Congress and Expo on Breast Pathology and Cancer Diagnosis & 20th International Conference on Medicinal Chemistry and Rational Drugs
Antonio Feula
Myokardia, USA
ScientificTracks Abstracts: J Clin Exp Pathol
Abstract
Room temperature iodocyclisation of homoallylamines stereoselectively delivers functionalised 2- (iodomethyl)azetidine derivatives in high yield. Increasing reaction temperature from 20�°C to 50�°C switches the reaction outcome to realise the stereoselective formation of functionalised 3-iodopyrrolidine derivatives. It was shown that these pyrrolidines are formed via thermal isomerisation of the aforementioned azetidines. Primary and secondary amines could be reacted with iodomethyl azetidine derivatives to deliver stable methylamino azetidine derivatives. With subtle changes to the reaction sequences homoallyl amines could be stereoselectively converted to either cis- or trans- substituted 3-amino pyrrolidine derivatives at will. The stereochemical divergent synthesis of cis and trans substituted pyrrolidines supports an ion part, aziridinium, isomerisation pathway for azetidine to pyrrolidine isomerisation. Six azetidine derivative were probed in a zebrafish embryo developmental assay for capacity to illicit morphological changes. The range of effects across the probed molecules demonstrates the suitability of this assay for screening azetidine derivatives. One of the probed molecules, exhibited particularly promising effects in the developmental assay.Biography
Antonio Feula is a PhD Chemist with 10 years of research experience in organic multi-step synthesis, drug discovery, surface chemistry, medicinal and supramolecular chemistry. Expertise ranges from multi-step synthesis (designing and performing) to chromatography (sample isolation, purification) and NMR methodologies. He has been part of several cross-functional teams including engineers, chemists and biologists. Antonio was a post-doctoral research fellow at the University of Reading and Oxford before moving to USA where he worked for Merck, Coty and Orthobond. He is currently involved in the drug discovery of small molecules for genetic heart diseases at Myokardia in South San Francisco.
E-mail: afeula@myokardia.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi