Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
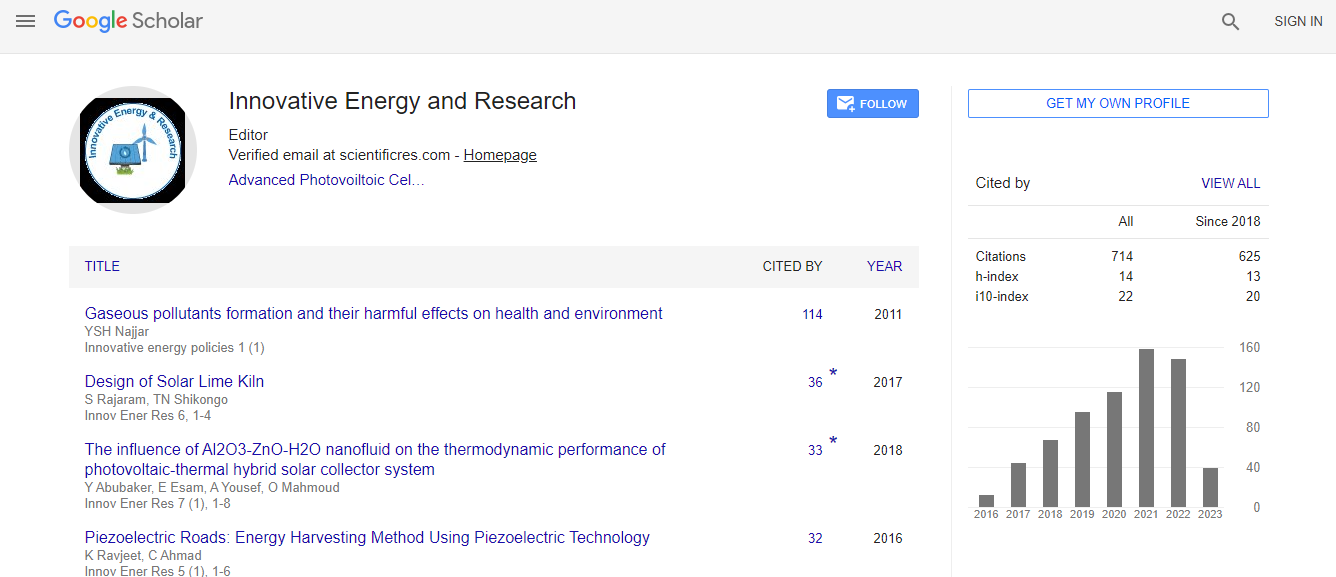
Google Scholar citation report
Citations : 712
Innovative Energy & Research received 712 citations as per Google Scholar report
Innovative Energy & Research peer review process verified at publons
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Superior hydrogenation/dehydrogenation kinetics of MgH2 nanopowders upon mechanical doping with amorphous Zr2Ni
20th International Conference on Advanced Energy Materials and Research
Fahad Al-Ajmi
Kuwait Institute for Scientific Research, Kuwait
ScientificTracks Abstracts: Innov Ener Res
Abstract
Hydrogen is an energy carrier, which holds tremendous promise as a new clean energy option. Hydrogen storage, which is considered to be the most important factor cutting across both hydrogen production and hydrogen transportations, has been the subject of intensive research for many years. Mg and Mg-based materials have opened promising concept for storing hydrogen in a solid-state matter. The natural abundance, cheap price, operational cost effectiveness, light weight, and high hydrogen storage capacity (7.60 wt.%, 0.11 kg H2L−1) are some advantages of Mg and Mg-based alloys making them desirable storage materials for research and development. Whereas all catalytic materials used to improve the behaviors of hydrogenation/dehydrogenation kinetics for MgH2 have long-range order structure, the present work proposes two different types of structure; i.e. short range- and medium range- order. For the purpose of the present study, MgH2 powders were prepared by reactive ball milling of Mg powders under 50 bar of H2, using room-temperature high-energy ball mill [5]. Ultrafine powders of amorphous- and big cube-Zr2Ni phases were prepared by ball milling small bulk pieces of tetragonal-Zr2Ni alloy prepared by arc melting technique. Small volume fraction (10 wt. %) of amorphous and big- cube powders obtained after ball milling for 100 and 150 h, respectively were individually mixed with as-synthesized MgH2 powders and then ball milled for 50 h. The results have shown that nanocomposite MgH2/10 wt.% metallic glassy Zr2Ni powders had high density of hydrogen (~6 wt.%) and possessed fast kinetics of hydrogen uptake/release at 250°C within 1.15 and 2.5 min, respectively. Whereas, MgH2/10 wt.% of big cube Zr2Ni nanocomposite showed moderate improvement on hydrogenation (1.8 min)/dehydrogenation (7 min) kinetics due to the heterogeneous distribution of their particles onto the MgH2 powders. Recent Publications: 1. El-Eskandarany, M. Sherif (2017) Synthetic nanocomposite MgH2/5 wt. % TiMn2 powders for solid hydrogen storage tank integrated with PEM fuel cell Nature, Sci. Rep. 7: 13296; doi: 10.1038/s41598-017-13483-0.www.nature.com/ scientificreports. 2. El-Eskandarany, M. Sherif et al. (2017) Structure, morphology and hydrogen storage kinetics of nanocomposite MgH2/10 wt% ZrNi5 powders, Materials Today Energy, 3: 60-71. 3. El-Eskandarany, M. Sherif et al. (2016) In-situ catalyzation approach for enhancing the hydrogenation / dehydrogenation kinetics of MgH2 powders with Ni particles. Sci. Rep. 6, 37335; DOI: 10.1038/srep37335 www. nature.com/scientificreports. 4. El-Eskandarany, M. Sherif (2016) Metallic glassy Zr70Ni20Pd10 powders for improving the hydrogenation/ dehydrogenation behavior of MgH2. Nature, Sci. Rep. 6, 26936; doi: 10.1038/srep26936. www.nature.com/ scientificreports. 5. El-Eskandarany M.Sherif, Shaban E., Alsairafi A. (2016) Synergistic dosing effect of TiC/FeCr nanocatalysts on the hydrogenation/dehydrogenation kinetics of nanocrystalline MgH2 powders. Energy 104: 158-170.Biography
Fahad Al-Ajmi works at Kuwait Institute for Scientific Research KISR in the Nanotechnology and Advanced Materials department. He obtained his master degree in advanced chemical engineering from the University of Manchester, and the bachelor degree in chemical engineering from Swansea University UK.
E-mail: ftajmi@kisr.edu.kw

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi