Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
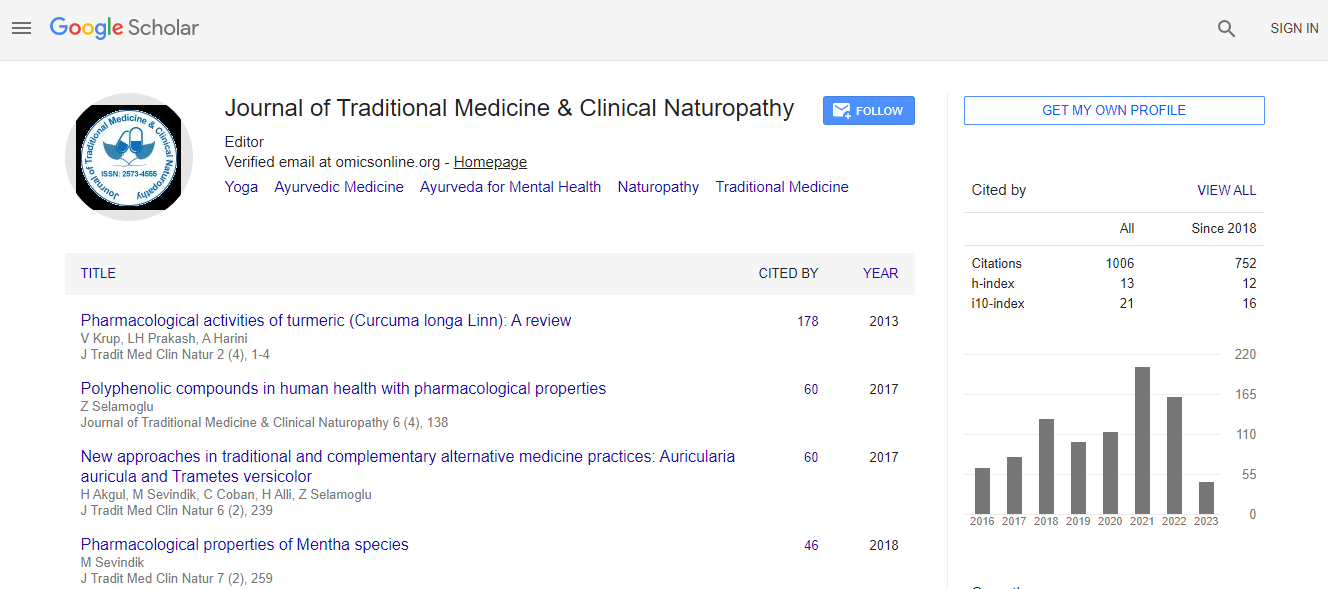
Google Scholar citation report
Citations : 1504
Journal of Traditional Medicine & Clinical Naturopathy peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Standardization and HPTLC fingerprinting of Unani compound formulation Sufoof Jawahir Mohra with modern analytical techniques
8th International Conference and Exhibition on Traditional & Alternative Medicine
Masroor Ali Qureshi, N M A Rasheed, Gulam Mohammed Husain, M H Kazmi and Mohammad Husain
Jamia Millia Islamia, IndiaCentral Research Institute of Unani Medicine, India
ScientificTracks Abstracts: J Tradit Med Clin Natur
Abstract
With global realization that use of synthetic drugs is not safe on the long run, the medical fraternity at large is looking at alternatives from natural sources to combat diseases particularly those in which conventional modern system of medicine has little to offer. This realization on the one hand has increased demand for herbal drugs and on the other hand need for quality standardization of drugs has gone up. The standardization of herbal drugs used in Unani system of medicine such as Sufoof Jawahir Mohra which is prescribed in Unani system for therapeutic use as tonic to multiple vital organs and stimulant to innate heat and it is useful in general weakness and weak functions of the vital organs has been taken up for standardization by modern analytical techniques, so as to ascertain its quality standard. The parameters which were carried out are organoleptic parameters, physicochemical parameters and high-performance thin layer chromatography revealing specific identities for the particular drug and to evaluate pharmacopoeia standards. Results suggest that the drug is safe for therapeutic use and the present study can be used as a reference standard for quality control in future.Biography
Masroor Ali Qureshi is a Scientist L-IV in Central Council for Research in Unani Medicine (Ministry of AYUSH, Government of India). He has 22 years of research experience in clinical research. He has published 34 papers in national and international journals and 15 papers in national and international conferences in India and abroad. Presently, he is pursuing PhD from Department of Biotechnology, Jamia Millia Islamia, New Delhi, India.
E-mail: doctormasroorali@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi