Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
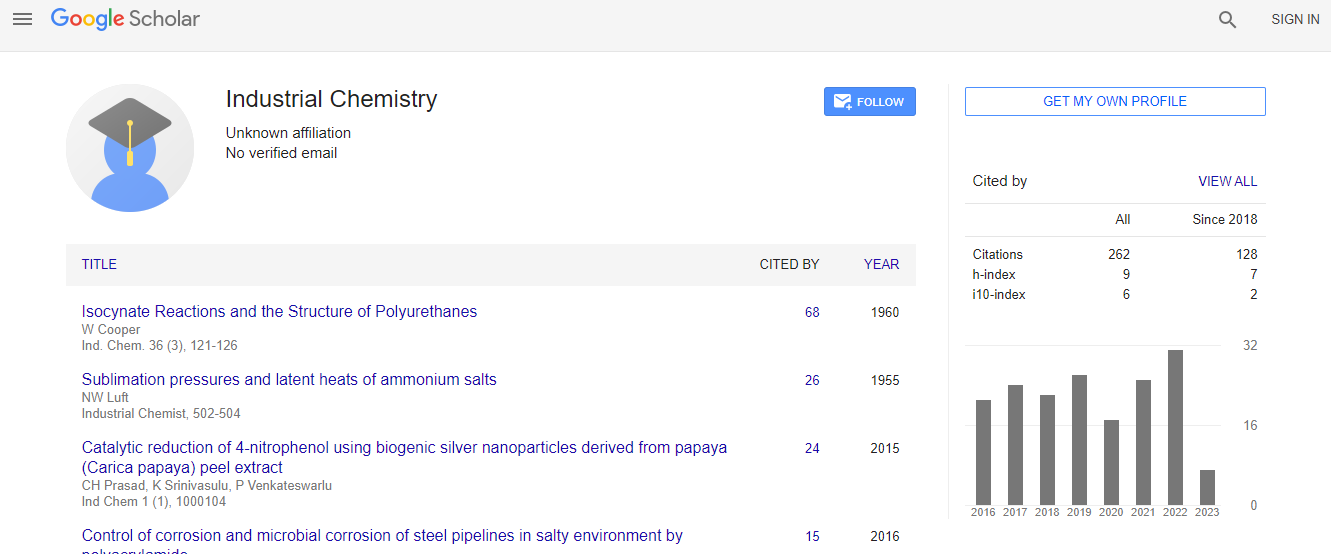
Google Scholar citation report
Citations : 262
Industrial Chemistry received 262 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Geneva Foundation for Medical Education and Research
- Euro Pub
Useful Links
Recommended Journals
Related Subjects
Share This Page
Small organic molecules as catalysts for asymmetric direct aldol reactions in aqueous media: A green chemistry approach for industrial applications
2nd World Conference on Industrial Chemistry and Water Treatment
Kartick C Bhowmick
University of Texas at San Antonio, USA
ScientificTracks Abstracts: Ind Chem
Abstract
Presently asymmetric organocatalysis in aqueous media is one of the most focused areas of research field in asymmetric synthesis. Asymmetric carbon-carbon bond forming reactions occupy the central area in the field of asymmetric organic synthesis where aldol reaction is the vastly studied one. A wide range of smart organic materials, including proline and its derivatives have been proved to be efficient catalysts for asymmetric aldol reactions. In recent years, more attention has been paid to develop organocatalysts for the asymmetric direct aldol reactions in water because it provides some unique properties, which include large cohesive energy density, very high surface tension, hydrophobic effect and most importantly it is environmentally benign solvent. The development of asymmetric organocatalyzed direct aldol reactions in aqueous media, for example, a very small organocatalyst, L-Proline hydrazide has been used for direct asymmetric aldol reaction of various ketones with aromatic aldehydes at room temperature in presence of several acid additives. A loading of 10 mol% of the catalyst and p-toluenesulphonic acid as additive was employed in this reaction, and good yields (up to 99%), with high anti/syn diastereoselectivities (up to 95:5) and enantioselectivities (up to >99.9%) could be achieved in aqueous media. Another new organocatalyst, derived from 4-hydroxy-L-proline and abietic acid was used for aldol reactions between substituted aromatic aldehydes and various ketones in presence of several acid additives in aqueous media. The corresponding aldol products were obtained in high isolated yields (up to 99%) with high anti-diastereoselectivities (up to 94%) and enantioselectivities (>99.9%). The catalyst loading was reduced to as low as 1 mol% only and very significantly, the aldol reactions were found to be extremely fast in water. In addition to the development of the above organocatalysts, the effect of several acid additives was investigated in asymmetric direct aldol reaction catalyzed by a C2-symmetric organocatalyst in aqueous media.Biography
Kartick C Bhowmick research focus on the development of asymmetric organocatalysis for carbon-carbon bond-forming reactions in aqueous media. His research on the development of small organic molecules as organocatalysts for direct aldol reaction impacted significantly in the field of asymmetric organocatalysis. He has developed many optically pure organic molecules which efficiently catalyzed the direct aldol reactions with great yield and selectivity in aqueous media. His newly developed methodology replaced the use of volatile organic solvents and hazardous metal catalysts in asymmetric aldol reactions, thus by contributing enormously towards the development of sustainable organic synthesis.
Email: kartickb@yahoo.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi