Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
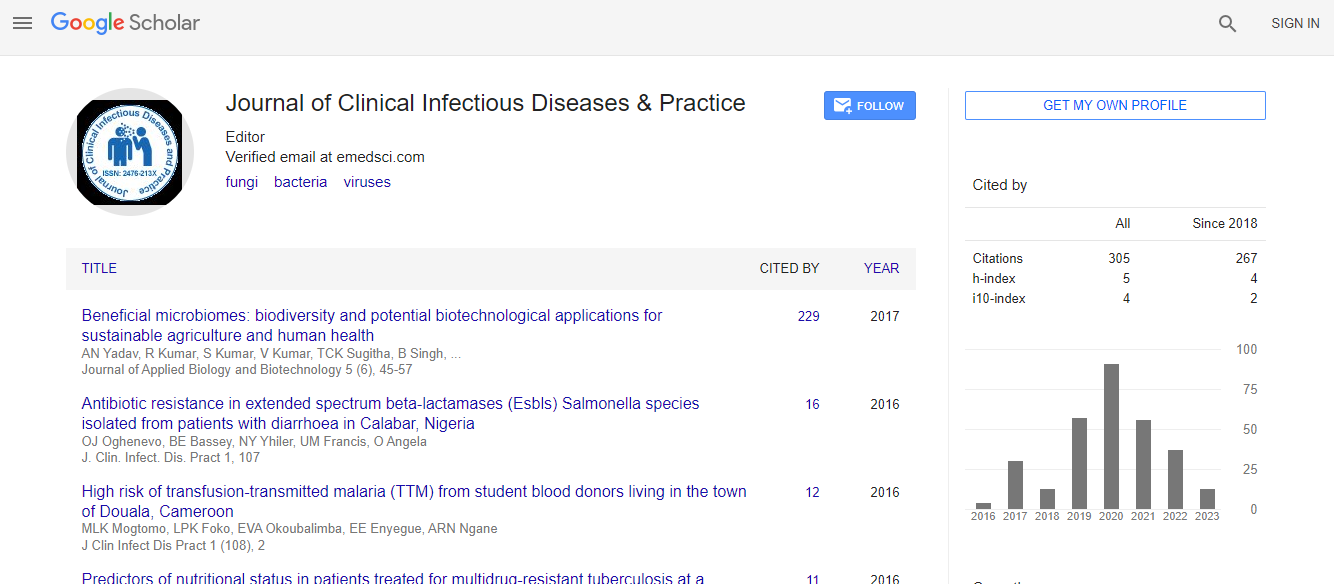
Google Scholar citation report
Citations : 531
Journal of Clinical Infectious Diseases & Practice peer review process verified at publons
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Share This Page
Red blood cell-encapsulated enzymes: An innovative therapeutic approach to overcome challenges of enzyme replacement therapies for rare diseases
9th World Congress on Rare Diseases and Orphan Drugs
Emmanuelle Cecile Dufour
Erytech Pharma, USA
ScientificTracks Abstracts: J Clin Infect Dis Pract
Abstract
Many inborn errors of metabolism (IEM) disorders are due to defects in single genes encoding key metabolic enzymes. In most cases, clinical manifestations of these disorders are driven by the over-abundance of a metabolite or the scarcity of an essential metabolite. Though rare, IEM disorders can have devastating consequences for patients and their families. While some Enzyme Replacement Therapies are commercially available for a few IEM disorders, the clinical benefits of these approaches are often outweighed by the emergence of hypersensitivity and the rapid clearance of enzymes. Therefore, there is a high need for better tolerated and longer-acting replacement enzymatic activity to alleviate the burden of IEM disorders. RBCs are the most abundant cell type in the human body and their biology is characterized by a long lifespan and access to all tissues and organs. Thanks to their biocompatibility and shielding properties, they can serve as a circulating bioreactor when loaded with enzymes. ERYTECH is a leader in RBC therapeutics. Its ERYCAPS® platform enables the encapsulation, at industrial scale, of active drug substances inside RBCs using hypotonic loading, which has been shown to maintain all the RBC functionalities. ERYTECH has demonstrated that RBC-encapsulated enzymes exhibit substantially improved in vivo performance vs. non-encapsulated enzymes, including extended enzymatic activity. Results from two early programs using enzyme-loaded RBCs in in vivo models for Arginase-1 Deficiency and Classical Homocystinuria will be presented. These promising results combined with ERYTECH’s extensive clinical experience with RBC therapeutics, support the possibility that RBC-loaded enzymes may provide superior safety and efficacy as compared with traditional ERT approaches for the treatment of IEM disorders.Recent Publications
1. Gay F, et al. (2017) Methionine tumor starvation by erythrocyte-encapsulated methionine gamma-lyase activity controlled with per os vitamin B6. Cancer Med. 6(6):1437-1452.
2. Bourgeaux V, et al. (2016) Drug-loaded erythrocytes: on the road toward marketing approval. Drug Des Devel Ther. 10:665-76.
3. Thomas X and Le Jeune C (2016) Erythrocyte encapsulated l-asparaginase (GRASPA) in acute leukemia. Int J Hematol Oncol. 5(1):11-25.
4. Yew N, et al., (2013) Erythrocytes encapsulated with phenylalanine hydroxylase exhibit improved pharmacokinetics and lowered plasma phenylalanine levels in normal mice. Mol Genet Metabol. 109(4):339- 344.
5. Bourgeaux V, et al., (2012) Efficacy of homologous inositol hexaphosphate-loaded red blood cells in sickle transgenic mice. Br J Haematol. 157(3):357-369.
Biography
Emmanuelle Cecile Dufour has obtained her PhD in Biochemistry and has been working with Erytech Pharma for 10 years. She is involved in the preclinical development of enzyme loaded-red blood cells as therapeutics for inborn errors of metabolism.
E-mail: Emmanuelle.dufour@erytech.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi